Arrhythmia—a cardiac disease in which the heart beats irregularly or at an abnormal pace—is caused by faulty electrical signal generation within the heart at the SA node. Recognizing the electrical properties of the heart, engineers created a treatment device, the artificial pacemaker, by applying principles of electrical engineering. The device controls the rate and rhythm of the heartbeat by overriding faulty natural signals with generated electrical pulses. The first pacemaker was created in the 1920s, and subsequent innovations in batteries, transistors, and microprocessors allowed the pacemaker to be portable, wearable, and eventually implantable. Today, pacemakers can be programmed for different rates and automatic regulatory functions, but they are still limited in battery life and have risks of infection or failure. Engineers are applying the concepts of piezoelectricity and ultrasound to address these problems, and the results are promising for a new generation of smaller, safer pacemakers.
Introduction
We like to think we have complete control over our bodies – looking, touching, and moving wherever we want. However, there is one thing we cannot systematically control with our minds: our heartbeat. Even when the brain is resting at night, the heart never fails to keep beating at the same rate. This is because the heart serves the critical function of facilitating blood circulation, which in turn supplies oxygen to different organs in the body. A timely and constant delivery of oxygen is critical for proper body function: our cells rely on oxygen to perform basic tasks like cellular respiration, the process that harvests energy to fuel cellular activities. The interruption of the body’s oxygen supply for even a few minutes can have catastrophic effects, like the death of brain cells [1]. One of the most common medical conditions that can lead to interrupted blood supply is arrhythmia, when the heart beats at an abnormal rate due to heart signaling malfunctions. To treat this problem, engineers applied principles of electrical engineering and created the artificial pacemaker, a device placed in the chest or abdomen that generates electrical pulses to control the rate and rhythm of the heartbeat. Since the first artificial pacemaker of the 1920s, pacemakers have undergone significant improvements over the years through the combined efforts of electrical engineers and medical doctors. Moreover, different engineering disciplines have now been applied to the development of the pacemaker, and these new techniques offer exciting potential for pacemakers of the future.
How the Heart Works
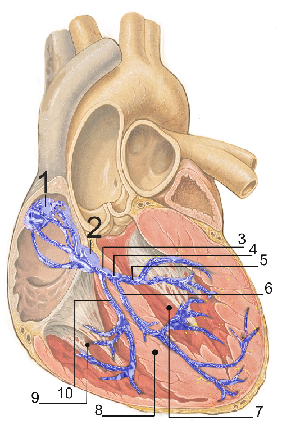
To understand the clever engineering behind artificial pacemakers, we must first understand how the heart works. The heart is a hollow and highly muscular structure that pumps blood throughout the blood vessels by repeated rhythmic contractions. In the average human with a resting heart rate of 72 beats per minute, 5 liters of blood are pumped through the heart every minute – roughly the total volume of blood in the human body [1]. Unlike most human organs, the heart is not controlled by the nervous system. In fact, the electric signals that trigger heartbeats originate in the heart itself. The heart contains autorhythmic cardiac muscle cells that contract and relax on their own, even outside the body. These cells form the sinoatrial (SA) node located on the top right chamber of the heart (the right atrium) and the atrioventricular (AV) node located in the wall dividing the left atrium from the right. These two nodes are pictured in the heart in Fig. 1.
The SA node (sometimes called the pacemaker) generates electrical impulses similar to nerve impulses at the start of every cardiac cycle, setting the rate and timing at which the rest of the heart contracts [1]. The impulses then spread rapidly through the top of the heart, causing uniform contraction in both atria. As the impulses reach the AV node (the “relay station”), they are slightly delayed and then conducted to the apex at the bottom of the heart. From here the impulses are spread throughout the ventricular walls, creating a strong contraction that pumps blood out of the heart into the rest of the body [11]. Depending on the different activities we perform, our body speeds up or slows down the heart’s tempo by regulating the SA node [1].
Abnormal Heartbeats: Arrhythmias
When the SA node doesn’t function correctly, the heart can beat too fast, too slow, or with an irregular rhythm. These problems are called arrhythmias and can be classified into three major subtypes: bradycardia (when the heart beats too slowly), tachycardia (when the heart beats too quickly), and fibrillation (when the atria or ventricles quiver instead of contract). When the heart doesn’t beat at the correct rate, blood and oxygen are not delivered effectively, and can result in fatigue, shortness of breath, or fainting. In severe cases, arrhythmias can cause severe organ damage, loss of consciousness, or even death [2]. Arrhythmias can arise from natural aging, heart muscle damage from a heart attack, medications, and several genetic conditions.
Over three million people with arrhythmia worldwide are treated with an implant device called the artificial pacemaker [3]. A pacemaker generates regular low energy pulses that overcome the faulty electrical signaling from the SA node, prompting the heart to beat at a normal rate. Even though the concept behind pacemakers is straightforward, the execution is not as easy.
The Evolution of Pacemakers
Although the correlation between electricity and the human heartbeat had been found as early as the 1800s, electro-stimulation devices for the heart did not appear until a century later [4]. The very first pacemaker was developed in the late 1920s almost simultaneously by both Australian anesthesiologist Mark Lidwell and American physiologist Albert Hyman. In 1928, Lidwell created an external device that delivered alternating current through a needle that was inserted directly into the patient’s ventricle. In 1932, Hyman created a similar device he named “artificial pacemaker,” a term still used today. The device was intended to restore a normal heartbeat to patients whose hearts had stopped. It consisted of a large, hand-wound current-generating motor and a bipolar needle that encompasses both negative and positive electrodes. The current generated was delivered through the bipolar needle into the right atrium (where the natural impulse originates) at rates of 30, 60, or 120 per minute. Despite his efforts, Hyman’s work faced many technical problems as well as opposition from the medical and social community. Hyman’s experiments were rejected by the Journal of the American Medical Association, no one agreed to manufacture the device, and some even regarded it as the work of the devil [4].
In the early 1950s, the first portable pacemaker was created by Canadian electrical engineer John Hopps [12]. The new pacemaker was a bulky external unit powered through household outlets, and it was considered “portable” since it could be wheeled around among different outlets. Instead of driving a conductive needle straight into the heart, Hopps’s device took the transvenous approach: electricity was delivered through a flexible bipolar catheter inserted through a vein into the right atrium of the heart. As a result, patients’ heart rates were successfully controlled without painful chest contractions. Nonetheless, the devices were not very desirable since they had low mobility and often contributed to other health complications. Because the pacemaker was external, infection often occurred in the chest along the lead [4].
In the late 1950s to early 1960s, several breakthroughs were achieved, eliminating the major problems with external pacemakers and shaping them into what they are today. Inspired by the rhythmic output of electronic metronomes, electrical engineer Earl E. Bakken and his brother-in-law (founders of the leading medical electronics company Medtronic Inc.) created the first battery-operated, wearable pacemaker [12]. The electronic metronomes used a circuit element called a transistor, which is a small semiconductor that switches signals on and off [5]. Bakken’s device combined the rather simple transistor-based circuits from the metronome with a miniature mercury battery that was able to supply power for around 1000 hours. This innovation significantly improved the size and mobility of pacemakers, allowing them to be worn on the neck or strapped onto the body of a patient [4].
Shortly after Bakken’s wearable pacemaker was developed, Swedish surgeon and engineer Ake Senning and physician Rune Elmqvist produced the first implantable pacemaker [4]. They made use of one of the first silicon transistors at the time, which significantly lowered the energy needed to power the pacemaker. In addition, they replaced the mercury battery with a nickel-cadmium battery that is rechargeable through an antenna [4]. This allowed the pacemaker to become the size of a Kiwi shoe polish can, small enough to be implanted in a patient’s chest [12]. (A Kiwi can, in fact, served as the mold for the first prototype of Senning’s and Elmqvist’s design.) Since then, the technology of implantable pacemakers for long-term therapy has developed rapidly with advancements in electronic technology, giving rise to the pacemakers available to patients today [4].
Modern Pacemakers
How They Work
Today’s artificial pacemakers have improved significantly from the early models. Modern pacemakers weigh less than an ounce, and they are only slightly larger than the size of a wristwatch face. In addition, they not only have the ability to pace heartbeats through electric currents, but they can also monitor the heart’s natural electrical activity [6].
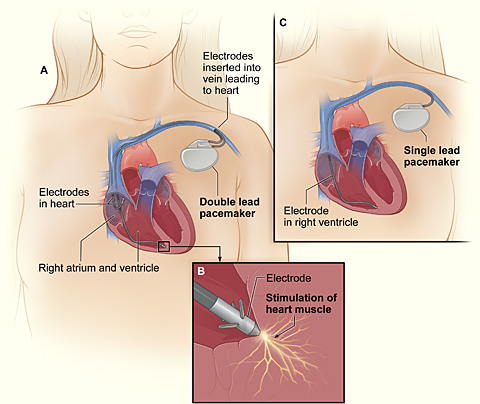
A pacing system has two major components, pictured in Fig. 2: the pacemaker and the pacing lead(s). The pacemaker unit consists of a small lithium battery (the generator), a microprocessor with memory and electrical circuits, a metal case, and a plastic connector block to which the lead is attached [7]. The pacing lead is a strong and highly flexible plastic-insulated wire designed to withstand the constant bending and twisting resulting from body and heart movements [6]. The pacing lead contains electrodes that act as both electricity conductors and sensors. The lead collects information about the electrical activity from the heart and sends it to the microprocessor in the pacemaker. The microprocessor, which is essentially a computer, records the heart’s electric activity and rhythm. If an abnormal natural electric pulse is detected, the computer directs the generator to create a small current that travels through the leads into the heart. In some cases, the pacemakers are also responsive to the particular rate of the heart’s beat: by sensing changes in blood temperature and breathing, the pacemaker can speed up or slow down the heart’s rate according to the activities that the patient is performing [7]. In addition, the pacemakers can be adjusted through a radio frequency remote control device called a “programmer.” By placing the programmer over the pacemaker, doctors can access recordings of the heart’s activity, reprogram the signaling rate, and check the status of the battery [6].
Where They Go
The surgical procedure to implant a pacemaker is rather simple, usually taking around one to two hours. The placement of pacemakers is usually considered a minor surgery, as only a minor incision and local anesthesia are needed [13]. Through the incision, one or more pacing leads connected to the pacemaker are inserted into a vein close to the collarbone and moved into the desired position in the heart with the help of an X-ray [7]. The pacemaker is then inserted into a pocket under the skin just below the collarbone where the incision is made.
The positioning of the leads depends on the type of pacing the patient needs. Pacemakers are capable of two types of pacing: single-chamber pacing and dual-chamber pacing. In single-chamber pacing, only one lead is placed inside the heart, either in the right atrium or the right ventricle. A lead placed in the right atrium is used to correct faulty signaling from the SA node, where natural impulses are generated. If placed in the right ventricle, the lead is used to correct faulty signal transmission at the AV node, which is responsible for propagating signals throughout the rest of the heart. (This problem with the AV node is a condition called heart block.) When both the SA node and the AV node are defective, dual-chamber pacing is needed. Dual-chamber pacing has two leads, one attached to the right atrium and one in the right ventricle. In the case of dual-chamber pacing, the microprocessor determines which of the two leads—if not both leads—will deliver an impulse depending on the readings delivered from the sensors [6].
The Future of Pacemakers
Although pacemakers have significantly improved over the years, they still cannot offer long-term solutions to conditions like arrhythmia. One of the major downsides of modern pacemakers is that they have a battery life of five to seven years. This lifespan is much better than the early devices, but it is still not ideal for patients who require long-term treatment. When the batteries run out, they have to be replaced surgically, which is not only expensive but also increases the risk of infection. Another downside is the difficulty associated with placing the leads. Placing leads can be extremely difficult in certain patients, such as those with cardiovascular diseases. If the leads fail, major surgeries with a high risk of infection may be required to remove them [9]. Nonetheless, several ongoing studies show great potential for solving these problems.
A recent study by the Department of Aerospace Engineering at the University of Michigan in Ann Arbor offers a promising solution to battery life limitations. Instead of running on lithium batteries, the proposed device uses piezoelectricity—electricity generated from motion, which in this case is the heartbeat. The idea was taken from a light unmanned aircraft that is powered by the vibrations of its own wings. Based on a wide variety of simulated heartbeats, researchers found that the energy harvested from heartbeats were ten times more than the energy required for modern pacemakers. The potential for power-harvesting devices is extremely exciting, since it promises a power source that is a smaller (half the size of the batteries currently in use) and allows pacemakers to be self-powering [8].
Research efforts have also been devoted to exploring possible replacements for pacing leads. Cambridge Consultants, a company based in England, has engineered a pacing system that eliminates the need for leads altogether. The device uses a miniscule electrode—only the size of a grain of rice—that receives electric signals wirelessly though ultrasounds. The size of the electrodes allows them to be implanted directly onto the heart with a catheter (a thin flexible tube). The pacemaker is implanted in the chest, and then it sends ultrasonic pulses that create vibrations in the electrode, which turns into a current to stimulate the heart. Though the device is still in research phase, it shows great potential after the successful completion of several clinical trials [10].
With the today’s rapid rate of technological evolution, there is no doubt that pacemakers will keep getting smaller and more resilient in the near future.