The introduction of antibiotics in the twentieth century has led to their widespread use, as they have become a prevalent class of drugs prescribed worldwide. A continuous demand for antibiotics has circulated throughout the medical community to treat ailments that range from the common throat infection to life-threatening staphylococcus infections. Increased usage of antibiotics by the public has been associated with a rise in drug-resistant strains of pathogenic bacteria, a trend that poses a large problem for the medical community. Multiple solutions that have been proposed to ensure the continued treatment of bacterial infections, including limiting the use of antibiotics and the introduction of new advancements to treat such infections.
Birth of a “Miracle Drug”
A slight fever, difficulty swallowing, chills, and a bright red throat — these are symptoms associated with a common throat infection caused by streptococcus, a pathogenic bacterium [1]. Up until the early 20th century, no effective treatments were available to treat an ailment that is now considered non-threatening in the public eye. Even when under the care of a physician, other bacterial infections involved serious complications and fatality in many cases. Now, such infections are easily cured by using antiobiotics (see Fig. 1), which were once considered a “miracle drug” in the years of World War II [2].
The roots of antibiotics trace back to Alexander Fleming, an English scientist who isolated a mold that inhibits the growth of bacteria. This mold would go on to become known as penicillin, the name of the derived metabolite that demonstrates the ability to breakdown bacterial cells [2]. From this point on, drug companies placed a large emphasis on the discovery of other molds and organic materials that could be used to derive and synthesize new antibiotics. Currently, antibiotics are the second most prescribed class of drugs in the United States [3]. Such a fact validates the significant impact of Fleming’s discovery on the current medical system.
The 21st century poses a new challenge for the medical community, to retain the effectiveness of antibiotics that are currently in use. The success of antibiotics is limited by the fact that increased use can strengthen the resistance of the particular pathogen that is being treated. With an improved understanding of bacterial cells and their ability to become resistant, scientists look to maintain the efficacy of antibiotics and develop alternative methods to treat pathogenic strains. In the coming years, researchers will have to balance the benefits of antibiotics with the looming threat of creating a “superbug” that no known antibiotic can treat.
The Mechanism Behind the Magic
Before looking at how bacteria become resistant, a basic overview of antibiotics will reveal their revolutionary mechanisms. Antibiotics can be classified by multiple properties, one of which depends on how they interact with a particular bacterium. Some of the most common ways antibiotics stunt the growth of bacteria include inhibiting the synthesis of the nucleic acids and proteins that make up the cell, or disrupting the cell wall and cell membrane. A step by step look at the interaction between penicillin and streptococcus will demonstrate the mechanisms behind how antibiotics actually inhibit growth.
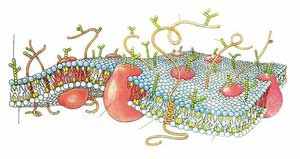
Penicillin is able to inhibit the functions of the bacterial cell membrane of streptococcus [4]. The cell membrane is described as a fluid mosaic model, a dynamic structure with a “mosaic” of various proteins embedded in or attached to a double layer of phospholipids, or fats [5]. Within this model, the proteins are able to move laterally along the membrane and are even able to move from the outside surface of the membrane to the inside and vice-versa. The picture below (see Fig. 2) demonstrates how these proteins traverse across the membrane (yellow). The cell membrane is the first barrier any cell has to foreign pathogens that are trying to enter the cell.
Penicillin acts on streptococcus by inactivating the enzyme transpeptidase, which is necessary for the cross linking of the cell walls [4]. In this interaction, the penicillin binds to a reception site on the transpeptidase protein, thereby blocking the entrance of a substrate that would normally bind to the enzyme and activate it. This blocking mechanism by Penicillin is known as competitive inhibition. Once attached, penicillin deactivates the enzyme, which causes cell wall construction to stop and the death of the bacterial cell. Many other antibiotics have similar mechanisms to that of penicillin, in that they are able to inactivate enzymes within the bacterial cell wall or other enzymes found inside the cell.
Failure of the Mechanisms
After over 70 years of use, antibiotics have maintained a relatively high success rate, but with the arrival of resistant strains of bacteria throughout the world this fact can change. Bacteria become resistant to antibiotics not by choice, but through random mutations in their chromosomal DNA. Such random mutations can result in circular pieces of DNA known as plasmids. In bacterial cells, plasmids that increase antibiotic resistance are called R plasmids. These extra DNA fragments can be transmitted between bacteria and are passed on to daughter cells during cell division [5]. The fact that these plasmids arise from random mutation within bacteria and can be easily transmitted between cells is what poses the largest problem for drug companies. Within a matter of hours, a mutated strain of a pathogenic bacterium can sweep through a hospital, leaving patients helpless if no antibiotics can effectively treat an infection by the new strain.
Working Against Our Evolution
Up to this point, hospitals and patients have depended on continual advancements in antibiotics to treat new strains of bacteria. Unfortunately, the long-term effects of such dependence could prove detrimental. To put this situation into perspective, one must realize that antibiotics have only been in use for less than a century, while bacteria have evolved and adapted to their environment for close to 3.6 billion years. This fact suggests an inability to determine whether advances in antibiotics can keep up with adaptations in pathogenic bacteria. There is no way to tell if the recent rise in resistant pathogens is a natural trend that will force companies to continually make stronger and more versatile antibiotics. If this trend does continue, there will be more than just the issue of actually developing the new antibiotics. More potent antibiotics could spell danger for the patient, as they could pose harsh side effects that make them difficult for practical use outside of a hospital setting.
Besides the issue of resistance, there is also the ethical dilemma posed by antibiotics in our society. In theory, not only are bacteria evolving (see Fig. 3), but so are humans from generation to generation. The difference between the two is that human evolution is not readily apparent because of the relatively long time span between generations. Research has shown that humans host a natural flora of bacteria that can help act as a barrier to pathogenic ones [5]. Throughout our evolution, humans developed not only these bacterial relationships, but also an immune system to protect the human body from pathogenic bacteria. The use of antibiotics prevents the human body from building up an acquired immunity to certain pathogens. This means the immune system of a particular individual is not given the chance to build up resistance to a pathogen as effectively when antibiotics are used. Rather, the individual becomes dependent upon antibiotics in the event that he is exposed to the same pathogen. The case of acquired immunity will only affect that particular generation, and in no way is passed on to the next generation genetically.
Along with acquired immunity, humans have been bestowed with innate immunity, a part of the immune system that is passed on from generation to generation and is not specific for particular pathogens [5]. In a way, innate immunity acts as the first response of the immune system to any pathogen. Innate immunity is non-specific, which means its mechanisms will attack any foreign material that enters the body. Acquired immunity is the second wave of defense, which must be built up to be effective against a particular pathogen. The medical community must question whether increased use of antibiotics and a dependence on them over our natural immune system could potentially have a negative effect on the ability of our body to fight infections. With pathogenic bacteria becoming increasingly virulent, we could enter a society where previously minor infections can no longer be handled by our immune system. Luckily, scientists have realized the potential side effects of antibiotics and are currently working on solutions to maintain the efficacy of antibiotics.
Limited Antibiotic Use and Emerging Alternatives
One of the main causes of antibiotic resistant bacteria has been the overuse of antibiotics by physicians and hospitals. Such overuse has been noted when patients are diagnosed with an upper respiratory tract infection, which could be either viral or bacterial. In the United States, a fifth of these infections are actually viral, but antibiotics are still prescribed [6]. To remedy this situation, medical campaigns have emerged that advocate a more conservative use of antibiotics. In order for such a movement to be successful, not only would doctors have to be more diligent in there diagnosis, but patients need to be accepting of the fact that every cold does not necessarily need a prescription for antibiotics.
Besides decreased usage, issues with resistant bacteria have sparked pharmaceutical companies to shift their research towards alternative medicines to treat infections. One example focuses on the idea of innate immunity, and how knowledge of this mechanism can lead to the development of new medicines. Currently, a project called Functional Pathogenomics of Mucosal Immunity is looking to gain a better understanding of the mechanisms behind innate immunity [7]. If they are successful, an entirely new set of medications would be unlocked to battle bacterial infections. Researchers in the project expect that through the mechanisms of innate immunity they could develop faster acting medicines that mimic the quick action of the immune system [7]. An example of innate immunity could be the many different types of mucus created by the body and the role these play in protecting against entry of pathogens into the body. Within the mucus are molecules, such as lysozyme in tears, which are able to break down bacterial cells before they have the chance to enter the body. By using our knowledge of these mechanisms, a revolutionary field of medicines is on the verge of being discovered. This research reveals how the negative aspects of antibiotics have led to positive advancements within the medical field, as the demand for new solutions to antibiotic resistant bacteria increases every day.
Conclusion
In less than a century, antibiotics have completely reshaped health systems and improved the ability of the medical community to treat patients with various ailments. Now, we have reached another turning point that may call for a radical change in how our medical system functions. In the near future, the scientific community and pharmaceutical companies will have to mediate the distribution of antibiotics in order to maintain their effectiveness. Continued research into both different antibiotics and new ways to deal with bacterial infections will be vital to the welfare of the public. Despite the fall back, it is the same spirit that led Alexander Fleming to his discovery in 1929 that drives the current research being done to reach even greater advancements in modern medicine.