In the Fall of 2007, Stephanie Angkadjaja was a sophomore majoring in Chemical (Environmental) Engineering. She likes watching football and basketball, collecting sneakers, and traveling to new countries.
Germs are everywhere, so it’s no surprise that antibacterial soaps, hand sanitizers, and lotions are as well. Despite this, consumer knowledge of Triclosan (the active ingredient in many antibacterial products) remains vague at best. A biochemical explanation of how Triclosan disables bacteria will give consumers a more scientific understanding of a product they use so often. After a closer look at how bacteria develop resistance to Triclosan and other antibacterial agents, consumers will learn that the ingredient is a double-edged sword. Will consumers want to continue using antibacterial products at the risk that those products will no longer be effective? Consumers will also learn of the adverse reactions Triclosan has with water to form chloroform, a possible carcinogen, and with sunlight to form dioxins, known endocrine disruptors. Because of the negative trade-offs associated with Triclosan, consumers should consider antibacterial products that do not use Triclosan and instead use ethyl alcohol as the active ingredient. Washing hands with plain soap and water the old-fashioned way is another reliable option.
Ewww! Germs!
In the 2004 film The Aviator, Leonardo DiCaprio portrays Howard Hughes, the famed 20th century Renaissance man with obsessive-compulsive disorder and a general paranoia of all things unclean. Scenes in the film show Hughes repeatedly washing his hands; the real-life Hughes, who lived in the mid-1900s, was known to pick up objects with paper towels in order to avoid germs. Today, pharmaceutical companies and the personal hygiene industry cater to the public’s similar obsession with cleanliness by marketing antibacterial soaps, gels, and lotions (see Fig. 1). The U.S. consumer demand for antibacterial products is projected to increase $8 million from 2005 to $136 million by 2009 [1].
What is the common active ingredient in these products that is popularly claimed to kill “99.9% of germs,”and how does it work? The secret weapon is Triclosan, a potent synthetic chemical. Although effective, the controversial chemical ironically contributes to bacterial resistance, decreasing its future effectiveness against bacteria. Potentially toxic side effects linked to Triclosan have also raised concerns among environmental protection and consumer advocacy groups. Consumers must be aware of the trade-offs associated with using Triclosan-based products.
A Quick Overview of Triclosan
Triclosan is classified as a broad-spectrum antimicrobial agent because it is effective against a wide range of microbes; the term antimicrobial implies that the chemical is antibacterial, antifungal, and antiviral [2]. Because Triclosan is extremely powerful, very low concentrations can effectively kill bacteria. For example, Colgate toothpaste lists 0.30 % as the percentage of Triclosan on their label. Although the percentage is almost negligible, 0.30 % is sufficient to qualify Triclosan as an active and powerful ingredient. In addition to soaps, Triclosan is found in mouthwashes, face washes, deodorants, toothpastes, and many other household products [3].
Biology 101
Understanding how Triclosan functions requires some rudimentary knowledge of biology, namely the interactions of cell membranes, fatty acids, and enzymes.
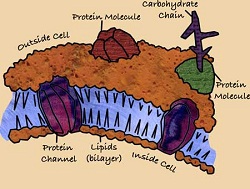
The cells of all organisms, including bacteria, require a cell membrane to survive. The cell membrane is a critical barrier that selectively allows oxygen, nutrients, and wastes to permeate and leave the cell [4]; it is the “edge of life, the boundary that separates the living cell from its nonliving surroundings” [4]. Without a permeable cell membrane, a cell would simply die. For example, wastes would not be transported out of the cell, causing toxins to accumulate and poison the cell. Curiously, it is precisely this function of the cell membrane that Triclosan is engineered to immobilize. Fig. 2 shows the important components of the cell membrane, including the lipid bilayer and proteins embedded in the bilayer that facilitate transport in and out of the cell.
The lipid bilayer holds the cell membrane together. Lipids, or fatty acids, are long hydrocarbon chains essential to cell membrane growth and function. Triclosan immobilizes the cell membrane by stopping the process that elongates these chains.
Triclosan stops the fatty acid elongation process by inhibiting a bacterial enzyme. An enzyme is a protein that catalyzes, or speeds up, a chemical reaction. In a catalyzed reaction, the reactant molecule is called a substrate. The substrate binds to the enzyme’s active site, which is shaped like a pocket or groove. The binding can be thought of as a handshake, a key entering a lock [4], or puzzle pieces fitting together. Competitive inhibitors are molecules that “steal” the spots of designated substrate molecules [4]. Once applied to the hands in the form of antibacterial soap, Triclosan acts as a competitive inhibitor, and a battle between Triclosan and bacteria for active sites ensues.
By stealing active sites from the natural substrate, Triclosan systematically kills bacteria by stopping fatty acid chain growth. This, in turn, stops the growth of the cell membrane and effectively kills the cell. The process is efficient, insidious and almost perfect, and when given the opportunity, Triclosan is extremely successful. The following description of how Triclosan works should be more meaningful now that relevant terms and processes have been introduced.
How Does Triclosan Work?
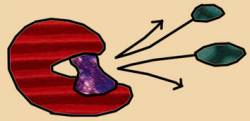
Fatty acid synthesis in bacteria is a multi-reaction process with different enzymes catalyzing each chemical reaction. One gene encodes one enzyme; Triclosan interferes with the gene that regulates the entire process. For example, in E. coli and M. tuberculosis bacteria, the regulatory gene, named FabI, encodes the enzyme called enoyl-acyl carrier protein reductase (ENR) [5]. Triclosan acts as a competitive inhibitor when it binds to the active site of the ENR enzyme and forms a FabI-NAD+-triclosan complex (see Fig. 3) [6].
Triclosan steals the active site on the ENR enzyme to form a complex never intended by the regulatory gene. The complex is analogous to a defected product in a factory. The formation of this complex signals Triclosan’s success. The alternate complex is unable to continue in the process of fatty acid synthesis, effectively ending the elongation of the fatty acid.
Construction and maintenance of the cell membrane relies on synthesis of fatty acids. Triclosan impedes fatty acid synthesis in bacteria and therefore weakens the cell membrane and effectively stops bacteria from functioning properly.
Resistance Issues
Bacteria can develop resistance to certain substances, especially those substances to which the bacteria are continually exposed. This trend has been seen over the past few decades with strains of infectious bacteria that develop resistant to antibiotics after continual administration of the same drug to a patient.
Triclosan contributes to bacterial resistance against antimicrobial agents because the substance does not completely evaporate. Ironically, this chemical imperfection is a consumer advantage; Triclosan-based antibacterial products are praised for their ability to stay on the skin and serve as an extra defense mechanism [3]. Beyond Pesticides, a consumer advocacy group, claims that Triclosan does “linger in the environment” [7]. Frequent use of antibacterial products adds to the amount of leftover Triclosan that bacteria can adapt to: “rapid evolution of antibiotic-resistant strains of pathogenic bacteria is a serious health threat aggravated by imprudent and excessive antibiotic use” [4]. Considering the fact that bacteria reproduce within minutes or hours, many generations of offspring can inherit favorable mutations and evolve into strains immune to Triclosan and similar antimicrobial agents [4].
The present classification of Triclosan as a nonspecific agent that interferes with random bacterial genes is now strongly debated because multiple research studies have confirmed that Triclosan does target a specific gene in bacteria [6]. For example, in E.coli,[/] the targeted gene is FabI. Bacteria can develop resistance to Triclosan if missense mutations are encoded in the targeted gene [6]. Although missense mutations change the coding of a gene, the protein created from these mutated genes will still function, but may not be recognized by a specific inhibitor [4]. As a result, the gene encoded by a missense mutation could be unrecognizable to Triclosan. This is especially problematic because bacteria can more easily develop resistance when only one non-random gene is targeted.
Bacteria can fight back against Triclosan by producing more of the substrate competing with Triclosan. This will result in Triclosan being outnumbered by substrate molecules and thereby preventing the formation of fatty acid synthesis-ending complexes. Another mechanism of resistance is an efflux pump that expels Triclosan out of the bacterium[3]. Most bacterial efflux pumps are nonspecific, meaning that Triclosan is blindly expelled along with other substances. Bacteria can also acquire resistance in one of three ways: transformation, conjugation, and transduction. Transformation occurs when a bacterium uptakes foreign genes, conjugation occurs when two bacteria directly exchange genes, and transduction occurs when a virus inserts genes into a bacterium [4]. One of these acquired genes could be a resistance gene.
Because resistance can be developed or acquired in many ways, there is an increased probability that the current form of Triclosan will no longer be effective. Triclosan will have to be reengineered to outpace bacterial evolution.
The Dangerous Side Effects
Most antibacterial products are used in homes and subsequently deposited in residential drains. Triclosan reacts quickly with chlorine in water, and only very low concentrations are required to form significant amounts of chloroform [8]. Chloroform is a suspect carcinogen that affects the central nervous system and causes liver and kidney damage [9]. The Triclosan-chlorine reaction is common in wastewater treatment plants, but is not considered as dangerous because of scale: the effects of the reaction in residential homes are multiplied [8]. Although toxicological tests have deemed Triclosan safe for human use in high concentrations on the skin, accumulation of Triclosan in water can cause a potentially toxic buildup of chloroform [10].
A research study done by Johns Hopkins University estimates that about 75% of Triclosan and similar chemicals deposited in residential drains eventually survive wastewater treatment at sewage plants [10]. While in the environment, Triclosan can also react with sunlight to form toxic organic compounds called dioxins. Dioxins, like Triclosan itself, are chemically stable, allowing them to accumulate in the body [11]. The effects of dioxins disrupt the endocrine system and the immune system. In addition to the direct hazards to humans, excess Triclosan may also kill beneficial bacteria [10].
An Alcohol-Based Antibacterial Option
Some antibacterial products, such as hand sanitizer (see Fig. 4), use ethyl alcohol instead of Triclosan as the active ingredient. Like Triclosan, ethyl alcohol kills bacteria by disassembling bacterial cell membranes (“FAQ”). Although alcohol-based products are not as effective as Triclosan-based products, the advantage of ethyl alcohol over Triclosan lies in its ability to evaporate completely so that none of the chemical is present for the bacteria to adapt to. Therefore, resistance issues are nonexistent in the use of ethyl alcohol as an antibacterial. Moreover, ethyl alcohol does not have the toxic environmental effects of Triclosan.
The Right Way To Wash Your Hands
Interestingly, it is possible to kill germs without using antibacterial products. With regular soap and warm water, the key is to wash hands long enough. Rather than rinsing immediately after applying soap to hands, rub hands together for at least fifteen seconds before rinsing. Dry hands before turning off the faucet. Use a paper towel to turn off the faucet in order to avoid germs on the faucet [12].
The Future
Currently, there is no government agency that monitors the amount of Triclosan and similar chemicals that is washed down residential drains and then into the environment. Many scientists, consumer advocacy groups (such as Beyond Pesticides), and even individual consumers growing increasingly aware of the dangers posed by Triclosan are pushing the Environmental Protection Agency to monitor and explore the extent of Triclosan accumulation in the environment [10].
Using antibacterial products provides reassurance to the Howard Hughes in everyone. But continued use of Triclosan-based products inadvertently helps bacteria develop resistance to Triclosan and other antimicrobial chemicals. In response, a second generation of Triclosan must be engineered to combat bacterial strains resistant to the first generation of Triclosan [6]. The second generation must be equally potent but nontoxic [13]. Naturally, bacteria will develop resistance to the second generation, and the uphill battle against evolution is repeated. In the meantime, washing hands with regular soap and water should help keep the germs away.
References
-
- [1] D. de Guzman. “SDA: Scrubbing Antimicrobial Myths.” Chemical Market Reporter, Vol. 269.4, pp. 31+, 2006.
- [2] A. Glaser. (2004). “The Ubiquitous Triclosan: a Common Antibacterial Agent Exposed.” Pesticides and You. [On-line]. Vol. 24.3, pp. 12-17. Available: http://www.beyondpesticides.org/pesticides/factsheets/Triclosan%20cited.pdf [May 26, 2006].
- [3] Ciba Specialty Chemicals. “Triclosan Information.” Internet: http://www.cibasc.com, Oct. 26, 2006 [Oct. 27, 2006].
- [4] N. A. Campbell et al. Biology. 5th ed. Menlo Park: Benjamin Cummings, 1999, pp. 93-517.
- [5] M. Kapoor et al. “Mutational Analysis of the Triclosan-binding Region of enoyl-ACP (acyl-carrier protein) reductase from Plasmodium falciparum.” Biochemical Journal, Vol. 381.3, pp. 735-741, 1 Aug. 2004.
- [6] R. J. Heath et al. “Mechanism of Triclosan Inhibition of Bacterial Fatty Acid Synthesis.” Journal of Biological Chemistry, Vol. 274.16, pp. 11110-11114, May 1999.
- [7] Macdonald, Veronica. “Household and Personal Care.” Chemical Week, Vol. 161.41, pp. 25-29, Nov. 2005.
- [8] K. L. Rule et al. “Formation of Chloroform and Chlorinated Organics by FreFe-chlorine-mediated Oxidation of Triclosan.” Environmental Science and Technology, Vol. 39.9, pp. 3176-3185, May 1, 2005.
- [9] Wikipedia. “Chloroform.” Internet: http://en.wikipedia.org/wiki/Chloroform, Oct. 19, 2006 [Oct. 26, 2006].
- [10] M. Cone. “Threat Seen From Antibacterial Soap Chemicals; The Compounds End Up in Sewage Sludge That is Spread on Farm Fields Across the Country.” Los Angeles Times Home ed., sec. A: 17, 10 May, 2006.
- [11] Wikipedia. “Dioxin.” Internet: http://en.wikipedia.org/wiki/Dioxin, Oct. 19, 2006 [Oct. 26, 2006].
- [12] P. Carroll. (2004, May). “Here’s the Dirt on Antibacterial Soaps.” Nurse’s Notebook. Nurse’s Notebook. [On-line]. Available: http://www.nursesnotebook.com/antibacterial_soaps.htm [Oct. 26, 2006].
- [13] M. Larkin. “A Close Look at Triclosan Raises Questions.” The Lancet, Vol. 353.9159, pp. 1160+, Apr. 3, 1999.