Abstract:
Brain-computer interface (BCI) technology is a communication system that offers a direct pathway between the brain and an external device. BCI systems decode neural signals to enable the control of prosthetic limbs, assistive devices, computer interfaces and more. This technology is beginning to revolutionize the way that healthcare providers can rehabilitate or augment the capabilities and quality of life of patients experiencing a wide range of neurological or psychiatric conditions. Taking an interdisciplinary approach of combining neuroscience, engineering, and computer science, researchers and developers have been able to create two avenues of the functioning for this technology: noninvasive and invasive BCIs. This article discusses the underpinnings of BCI’s functioning, the two different categories of the technology, and research to support the success of this emerging technology.
Imagine you wake up in the middle of the night with a throbbing headache. Trying to sleep it off, you go back to bed. In the morning, realizing that the pain hasn’t subsided, you attempt to call out to another person for help but find yourself unable to speak. Your vision blurs, and your body begins to tingle and eventually progresses to paralysis. An ambulance is sent to you, and you’re then treated at a local hospital. However, after hours of disrupted blood flow to your brain, you lose the functioning of your motor skills, rendering the movement of your upper-body useless.
This is an example of the long-term health impacts of a late response to a stroke.
Strokes are one of the many conditions now undergoing treatment with brain-computer interface (BCI) technology. BCIs are a communication system that can be used to send and receive signals between a person’s brain and an external device, such as a robotic limb for extremity movement or a computer for thought-translation. Today, healthcare providers can use noninvasive BCI methods to treat various conditions including strokes, developmental and psychiatric disorders, and degenerative diseases. Additionally, we are beginning to see the emergence of invasive methods for acute circumstances, such as for people who are deaf or blind.
Let’s take a deeper look into how this technology works and how it is beginning to revolutionize the world of patient care.
First, we need to unpack some basic neuroscience. It’s no secret that the human nervous system is complex, and to this day scientists still don’t know all the ins-and-outs of this intricate biological process. For newcomers to the field trying to understand this powerful organ, it can be quite shocking to find out that our brain runs on electricity. Taking advantage of our brain’s electro-chemical roots is the foundation of BCI. Thus, it’s important to have some basic knowledge of how neurons, the cells of our brain, work.
Some atoms possess charges: electrons carry a negative charge, protons carry a positive charge, and neutrons carry a neutral charge [15]. The way that these atoms combine create different chemical molecules that have slight electrical charges; these are known as ions [15]. If an ion has an excess of protons, it will be slightly positive, and vice versa. Much like magnets, opposites attract with ions. Two ions with opposite charges will attract each other, and those with like charges will repel. Within our nervous system, these ions can be found in our neurons and outside in the surrounding fluid [15].
All cells have a barrier called a cell membrane which surrounds them and protects their contents from the outside environment. Neurons possess a semi-permeable membrane, defined by only allowing certain ions to pass through depending on their characteristics. Neurons have a slightly negative resting potential due to the large negatively charged protein molecules in the intracellular fluid [15]. This means that there is an electrical potential difference, or voltage gradient, between the inside and outside of the neuron.
When a neuron is activated, it receives another neuron’s output and changes the permeability of its membrane. Smaller positively charged molecules flow in as they are attracted to the negatively charged ions within the cell [15]. This gradual influx of positive ions slowly changes the overall charge of the neuron, in a process called depolarization [15]. As the cell becomes increasingly positive, the membrane will allow more and more positive ions in, until for a moment, the cell possesses an overall positive charge or “action potential” that allows a neuron to “fire” [15].
Neuronal firing occurs within milliseconds across many neurons when we have a thought, for example. These thoughts are recorded as electrical signals in a BCI device. Thus, it is said that the BCI system works to decode the encoded intentions of a user that are held in these neural signals.
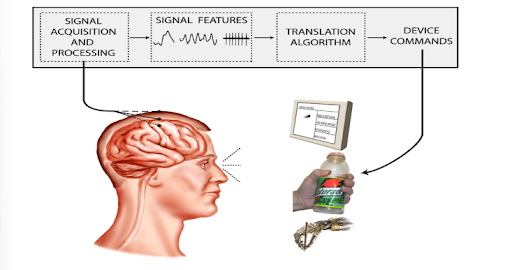
Figure 1. Illustration of BCI Technology Components [7]
Now that we know a bit about the basic functioning of the brain, we can discuss how BCI technology takes advantage of this electric communication system.
BCI systems are typically composed of four components: signal acquisition, signal processing, an output device, and an operating protocol [7]. These elements work to break down the brain’s electrical signals (input) to produce the user’s intended device commands (output). Thoughts are interpreted by a computer, translated using software, then used to initiate device commands. It may seem like this technology is a sci-fi mind-reading machine, however that is a big misconception. Brain-computer interfaces cannot extract thoughts from any unsuspecting person, rather a user and the BCI system work together. The system begins with a neuroimaging technique – a process of producing images of the structure or activity of the brain [18] – and the user thinking about an intention.
This technology can be divided into two main categories: noninvasive and invasive methods for decoding neural signals. Their differences lie largely in the type of neuroimaging technique used: invasive and noninvasive BCI. Noninvasive BCI captures brain activity externally; made possible by brain-scans like electroencephalography (EEG) and other noninvasive neuroimaging techniques. EEG has been a more widely used technique because of its inherent advantages: ease of use, low cost, ability to provide decently detailed information of the brain, and potential for movement of the user while wearing the device [10]. A patient’s neural activity is recorded from the scalp via electrodes. These are small metal discs attached to a thin cap that measure voltage changes that occur when our neurons fire (Fig. 2). This means that scientists or physicians can get inside your head, without being inside your head at all!
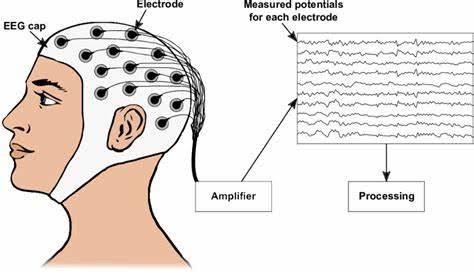
Figure 2. Illustration of EEG Cap on a Human Subject [12]
In contrast, invasive BCI records neural signals internally, via surgical implantation of the electrodes and placing them close to a target, or “problem,” area on the brain or spine [14]. This method relies on different neuroimaging techniques, such as electrocorticography (ECoG). Recall the noninvasive EEG technique, ECoG is a type of intracranial EEG. This means that like EEG, ECoG uses electrodes to record neural activity. However, this is done through implantation of those electrodes rather than recording from the scalp.
Invasive BCI, arguably, has greater advantages for patient care: it can obtain neural signals with much higher resolution. Electrodes placed extremely close or directly on a target area allows them to record brain activity much more clearly due to their proximity. The ability to place this device so close to an intended brain region supports accurate decoding of brain activity by a computer [14].
Think of it like trying to listen to someone talking. If you are in a different room from the person speaking, there’s a wall in the way; this is EEG. ECoG is like being in the same room as the person speaking. It is much easier to hear and understand the person if you are standing right in front of them, rather than through a wall (the scalp, skull, etc.).
EEG-based BCIs acquire a brain signal in two ways: the evoked or the spontaneous pathway. The evoked pathway requires some external stimulation of the user, such as visual or auditory stimuli, as well as intense focus on a single point [3]. For example, a healthcare provider might show pictures or a video that the user will have to continuously focus on. This technique aims to “evoke” a specific neural response. The spontaneous pathways on the other hand, require no external stimulation. This EEG method is based solely on the analysis of a user’s mental tasks carried out at their own free will, such as looking around the room or raising a hand [16]. Picking up on the brain signals from either of these neural pathways gives noninvasive BCI systems the data they need to help users control prosthetic limbs or a robotic exoskeleton.
Invasive BCI works similarly, however as previously mentioned, the brain signals are acquired internally using ECoG neuroimaging techniques. One major caveat of this method is the associated risks of infection, inflammation, tissue damage, or other complications of implantation during neurosurgery. Or worse, one wrong move, and you risk permanently destroying the functioning of some area of the brain.
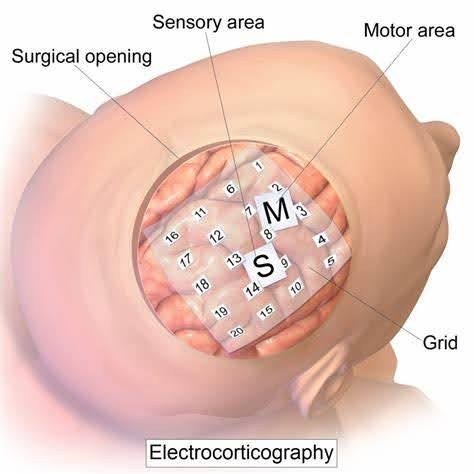
Figure 3. Illustration of Example Surgical Placement of ECoG on the Human Brain [21]
Regardless of the location of the electrodes, the function of the BCI system remains the same. Using EEG or ECoG, the electrodes will record the electrical signals of the brain, which will then be amplified, as well as filtered to get rid of any “noise” – unwanted interferences or variations in the signal [17]. After this extraction, the features of the signal should be much clearer, having a strong correlation with the user’s actual intentions [19]. These refined signals will be sent to a computer to be analyzed by a signal processing algorithm. In EEG, this is done through wire connections from the electrodes to the computer. In ECoG, this is achieved through frequency signals. The processing algorithm will translate the features of the signal and convert them into the appropriate commands (user’s intentions) [19]. Finally, the computer will send this information to the output device to guide its operation. This provides the device with all its necessary functions such as controlling a cursor on a computer screen or grasping a bottle with a robotic hand (Fig. 1) [19].
Whether noninvasive or invasive, we are beginning to see dramatically improved outcomes for patients using BCI technology. Future potential applications of BCI include brain-controlled wheelchairs, robots, or exoskeletons for amyotrophic lateral sclerosis (ALS) patients, stroke and trauma rehabilitation, and much more [5].
One clinical trial assessed the effects of BCI combined with mindfulness therapy on rehabilitation for patients that experienced single-sided paralysis following a stroke. The investigators assigned patients to either a control group that received conventional rehabilitation treatment or an intervention group that also received conventional rehabilitation in addition to noninvasive BCI training and mindfulness therapy [11]. The experiment found that participants assigned to the intervention group “showed statistically significant improvements in limb motor function, mindful attention awareness, activities of daily living, sleep quality, and quality of life” compared to the control group [11].
Invasive BCI, though much less studied due to the intense nature of the procedure, has also been successful on patients. One case study aimed to treat a patient with a cervical spinal cord injury that resulted in paralysis [20]. The patient received an ECoG implanted device over the area of the brain responsible for sensory and motor control. The researchers aimed to determine whether the invasive BCI system was capable of motor-imagery-initiated walking with an assistive walker [20]. The patient imagined taking a step to try and induce a stepping motion of the wearable robotic exoskeleton on their legs. Repeated thinking about moving each leg produced a specific set of neural signals. These signals were interpreted by a computer and sent to the robotic legs to initiate movement. Ultimately, the team was able to use the BCI system with 84.15% accuracy to help the patient with their walking rehabilitation [20].
As alluded to previously, there are complications that can be associated with the implantation of a BCI device. This procedure is neurosurgical, making it tremendously intense for the patient. Because of the implantation’s direct contact with brain tissue, invasive BCI has the potential to damage nerve cells or blood vessels in the surrounding area both during surgery and post-operatively, increasing the risk of infection [1]. Another outcome could be the complete rejection of the device by the body’s immune system. This means that defense cells would treat the BCI device as foreign, and consequently threaten the health of the person. The body would form scar tissue around the device, gradually degrading the quality of the recorded brain signals, and thus the efficacy of the BCI system [1]. For this reason, invasive BCI methods are strictly used for acute patient circumstances.
The field of BCI is currently moving extremely fast and stands at the forefront of revolutionizing healthcare. This technology represents a remarkable fusion of neuroscience, engineering, and computer science. Its transformative potential lies not only in its ability to decode and interpret our brain signals, but also its ability to use these signals to create or restore meaningful actions to patients. As researchers continue to advance BCI’s capabilities, we truly stand at the brink of a new era of neurological care and a previously unimaginable avenue of restoring quality of life to patients.
References
[1] B. Maiseli et al., “Brain–computer interface: trend, challenges, and threats,” Brain Informatics, vol. 10, no. 1, p. 20, Aug. 2023, doi: https://doi.org/10.1186/s40708-023-00199-3.
[2] “Brain Computer Interface | Psychology Today,” www.psychologytoday.com. https://www.psychologytoday.com/us/basics/brain-computer-interface
[3] C. Baiano and M. Zeppieri, “Visual Evoked Potential,” PubMed, 2024. https://www.ncbi.nlm.nih.gov/books/NBK582128/#:~:text=Visual%20evoked%20potentials%20%28VEP%29s%20represent%20a%20valid%20electrophysiological
[4] C. Guger et al., “Editorial: Breakthrough BCI Applications in Medicine,” Frontiers in neuroscience, vol. 14, pp. 598247–598247, 2020, doi: 10.3389/fnins.2020.598247.
[5] D. Chen, K. Liu, J. Guo, L. Bi, and J. Xiang, “Brain-Computer Interface and Its Applications | Frontiers Research Topic,” www.frontiersin.org, Feb. 08, 2023. https://www.frontiersin.org/research-topics/29617/brain-computer-interface-and-its-applications
[6] F. Pichiorri et al., “Brain-computer interface boosts motor imagery practice during stroke recovery,” Annals of neurology, vol. 77, no. 5, pp. 851–865, 2015, doi: 10.1002/ana.24390.
[7] G. Schalk and J. Mellinger, Practical Guide to Brain-Computer Interfacing with BCI2000: General-Purpose Software for Brain-Computer Interface Research, Data Acquisition, Stimulus Presentation, and Brain Monitoring, 1st ed. London: Springer London, Limited, 2010. doi: 10.1007/978-1-84996-092-2.
[8] K. Cherry, “Different Parts of a Neuron,” Verywell Mind, 2019. https://www.verywellmind.com/structure-of-a-neuron-2794896
[9] “Noninvasive EEG-based control of a robotic arm for reach and grasp tasks,” www.youtube.com. https://www.youtube.com/watch?v=w6QEGeIKHw0
[10] N. Padfield et al., “EEG-based brain-computer interfaces using motor-imagery: Techniques and challenges,” Sensors (Basel, Switzerland), vol. 19, no. 6, pp. 1423-, 2019, doi: 10.3390/s19061423.
[11] P. Wang et al., “Effects of brain-Computer interface combines with mindfulness therapy on rehabilitation of hemiplegic patients with stroke: a randomized controlled trial,” www.frontiersin.org.https://www.frontiersin.org/journals/psychology/articles/10.3389/fpsyg.2023.1241081/full
[12] S. Nagel, “Figure 2.1: Sketch of how to record an Electroencephalogram. An EEG…,” ResearchGate, Dec. 2019. https://www.researchgate.net/figure/Sketch-of-how-to-record-an-Electroencephalogram-An-EEG-allows-measuring-the-electrical_fig1_338423585
[13] E. Panfiloff, “When and Who invented BCI (Brain-Computer Interface)? – EnhancingBrain,” enhancingbrain.com. https://enhancingbrain.com/when-and-who-invented-bci-brain-computer-interface/
[14] Z.-P. Zhao et al., “Modulating Brain Activity with Invasive Brain–Computer Interface: A Narrative Review,” Brain Sciences, vol. 13, no. 1, p. 134, Jan. 2023, doi: https://doi.org/10.3390/brainsci13010134.
[15] M. Lyons, N. Harrison, G. Brewer, S. Robinson, R. Sanders, and D. Upton, “Biological Psychology,” Sage, 2014, doi: https://doi.org/10.4135/9781473922648.
[16] J. Del, P. Millán, A. Ferrez, Buttfield, J. D. Millán, and C. Menon, “Non Invasive Brain-Machine Interfaces Final Report.” Available: https://www.esa.int/gsp/ACT/doc/ARI/ARI%20Study%20Report/ACT-RPT-BIO-ARI-056402-Non_invasive_brain-machine_interfaces_-_Martigny_IDIAP.pdf
[17] “Signal and Noise – Definition and examples,” Conceptually. https://conceptually.org/concepts/signal-and-noise#:~:text=The%20signal%20is%20the%20meaningful%20information%20that%20you%E2%80%99re (accessed Apr. 28, 2024).
[18] “Neuroimaging, n. meanings, etymology and more | Oxford English Dictionary,” Oed.com, 2023, doi: https://doi.org/10.1093//OED//8325901506.
[19] J. J. Shih, D. J. Krusienski, and J. R. Wolpaw, “Brain-Computer Interfaces in Medicine,” Mayo Clinic Proceedings, vol. 87, no. 3, pp. 268–279, Mar. 2012, doi: https://doi.org/10.1016/j.mayocp.2011.12.008.
[20] K. C. Davis et al., “Design-development of an at-home modular brain–computer interface (BCI) platform in a case study of cervical spinal cord injury,” Journal of NeuroEngineering and Rehabilitation, vol. 19, no. 1, Jun. 2022, doi: https://doi.org/10.1186/s12984-022-01026-2.
[21] “Intro to Brain Computer Interface,” NeurotechEDU. https://learn.neurotechedu.com/introtobci/