What if we could produce and sell animal-free meat? Though it may sound like an oxymoron, this may be completely possible within the next few decades. With new technologies that allow scientists to create masses of muscle cells in a petri dish, engineers must step up to convert this technology to the production plant. By utilizing chemical engineering techniques, traditional animal slaughtering may become a thing of the past, replaced with cruelty-free and environmentally-friendly in-vitro meat.
Introduction
A busy shopper pauses in the meat section of the grocery store, scanning the selections of chicken, beef, lamb and fish. Every shelf contains an automatic unit, similar to an old-school ATM, where the desired weight, shape and type of meat can be entered into the system. After deciding on ten ounces of a circular chunk of chicken, the customer receives the perfect portion of protein. The bright yellow labels on the packaging indicate that the meat is animal-free, produced locally, and has a minimal environmental impact. Sound like an alternate reality? This may, in fact, be the norm in the year 2050.
This scenario could be the result of mass-producing in-vitro meat for consumption. Though this may not sound palatable right now, the benefits of this technology would revolutionize the way that the average person eats. Once perfected, in-vitro meat production could tackle world problems such as malnutrition, pollution, natural resource deprivation, and population growth issues [1]. So what is the science behind this test-tube food, and how will it make its way onto our dinner plates?
How Does It Work?
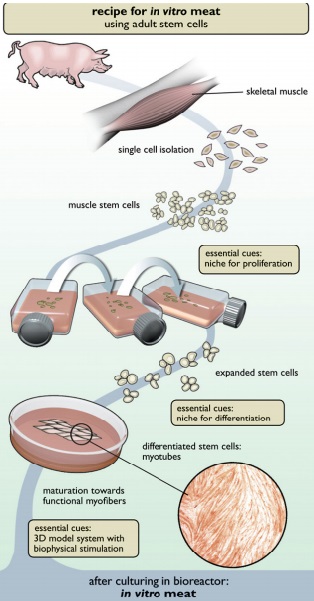
Cell culture methods for replicating cells do not involve a difficult process: most molecular biologists could hash out the steps in a few sentences. The process involves taking a small cell sample and incubating it in a petri dish with a growth medium until replication occurs. However, the process is not so simple when translated to meat production. Unfortunately, pig cells do not grow into a pork chop when simply placed in a petri dish as the beef patty in Fig. 1 might suggest.
The current process for in-vitro meat production follows a basic outline. Using the previous example, a biopsy of cells is taken from a pig with the goal of eventually producing edible meat. The myosatellite cells, which are the types of cell that becomes muscle, are extracted from this sample. A series of growth serums are added to the myosatellite cells, and they are then placed on a scaffold structure. This structure causes the cells to grow into fibers of muscle, instead of just masses of cells [2]. Then the fibers are stimulated by using “chemical signals from the animal’s body to . . . mimic the same electrical impulses” in order to bulk up the meat [3]. Lastly, the meat is processed to become sausage, pork or bacon. Although these ideas work in theory, there have been some issues with them in practice.
Limitations
Currently, almost every step of the procedure is causing difficulty. Firstly, isolating myosatellite cells is a lengthy process with low yield. It is also a crucial part of producing in-vitro meat. However, a recent study found a novel method for isolating these cells, which involved direct plating onto flasks coated with collagen. They found that “harvested chicken muscle cells showed the highest percentage of muscle satellite cells, and equine muscle cells presented the highest fusion index, an impressive ≈ 77%” [4]. This new method of isolation will allow for a better product down the line, as well as a more optimal procedure.
Although a solution to this problem has been found, this is only the beginning. One of the larger issues is the next step in the procedure: actually growing the cells to create the desired product. Growing strips of muscle tissue has been accomplished, but that is not the same as growing a cut of meat. To solve these problems, extensive research will have to be done on varying the types of growth media and scaffolding used. For example, an ideal growth medium would simultaneously deliver nutrients while removing waste, in addition to being cheap and plentiful. Ideal scaffolding “would have a large surface area for growth and attachment, be flexible to allow for contraction, maximize medium diffusion, and be easily dissociated from the meat culture” [5]. By experimenting with these variables, we will be able to find the optimal design for producing animal-free meat. However, if in-vitro meat does become available for mass consumption, it will likely not be created in small laboratories. So how do we transition into actual production? Simply put, we need “a systematic, if not systems, approach” to pushing in-vitro meat production to consumers [6].
Scaling It up with Engineering
The major barrier preventing the transition from the lab procedure (outlined in Fig. 2) to the factory is that large-scale tissue culturing is not widely practiced [5]. The closest concept is the bioreactor manufacturing in the biotechnology industry, but even that does not deal with the difficulties of sterility of muscle cells.
The first step of this bioreactor process involves a cycle of flow media through the bioreactor, similar to the function of the heart in the animal. The media concentration and flow rate, plus the optimal temperature and pressure for cell proliferation, would need to be adjusted depending on the system. Additionally, a method for recycling growth media—to clean it of waste from the bioreactor and to replenish the oxygen and nutrients—would need to be established. Unfortunately, current methods of “medium recycling [cause] a massive formation of cell aggregates accompanied by a progressive deterioration of the culture” [7]. Because the structure of the cells is so important to the final product, a recycled growth medium may not allow the cells to flourish properly, causing product loss. Most processes for recycled media are on a small-scale lab basis, rather than for a large-scale factory, so further research on this matter will depend on future developments in industrial engineering.
Next, at the bioreactor’s cell seeding center, the myosatellite cells would be attached to the tops of the scaffolds under a constant flow of oxygen. A three-dimensional system of delivering flow media and oxygen would be most optimal, rather than simply running these flows unidirectionally through the scaffold’s mesh. In this way, an ideal scaffolding system is one with flexibility and a large area.
The process of removing the completed cells from the bioreactor would first require sensors to estimate the growth of the meat and its point of completion. Once the bioreactor detects this, the scaffolding trays would then be removed and the cells would be extracted. Ideally, the bioreactor would yield a large piece of meat, which would then be sent off for conventional processing.
In the Future
Although we have been able to “explore the ways in which life becomes raw matter for engineering,” is in-vitro meat production capable of satisfying the consumer [8]? Would we be comfortable with a packaged mass of animal cells produced in a lab instead of the natural meat of an animal?
Chemically speaking, there isn’t a difference between meat from an animal versus from a test tube. But even so, it is highly possible that the public will not look favorably on a transition away from “natural” meat. In an article written by an applied philosophy research group, these opinions were tested in order to gauge reactions to test tube meat. It was found that “new types of arguments were considered relevant and ontological shifts [shifts in conceptions of the nature of being or existing] could even be seen to occur with what was considered ‘real meat’ and the ‘true nature’ of animal farming” [9]. Once the process is explained without the science-fiction-sounding aspects in-vitro meat, public opinions may slowly change. Especially when the benefits of this technology are explained, it becomes clear that this is a viable option. Most people like the idea of being able to “enjoy meat-eating while being good (or at least better) to the environment and reducing animal suffering” [10].
Conclusion
Currently, the world’s 1.5 billion livestock animals are responsible for between 15 and 24 percent of all anthropogenic greenhouse gasses—including 68 percent of ammonia, 65 percent of nitrous oxide, 37 percent of methane, and 9 percent of carbon dioxide” [11]. Our normal methods of meat-production are not sustainable, and we need a solution. This is where in-vitro meat comes in. Although there are many variables that must be optimized before full-scale production is possible, the benefits from this transition will affect the lives of every person in the world. Soon, you might even be reading this article over a nice bowl of animal-free chicken soup.