One of the continuing goals of scientists and engineers is to develop technologies which reduce the severity of physical disabilities. Hearing loss due to defects in the middle ear occur with great frequency, and the more severe cases can be treated with ossicular replacement prostheses. The main focus of this article will be on the development of these prostheses, including the various challenges engineers have encountered. The primary concern when developing these prostheses is the material to be used, since there are several criterion these materials must meet. Material availability has in turn had a major impact on the overall design of these prostheses, since the design must be based on the properties of the material used. These implants represent a major achievement in biomedical engineering, and are just one way in which engineers have had a major impact on the daily lives of many.
Introduction
It has long been the goal of scientists and engineers to develop technologies which render severe physical disabilities manageable or even negligible. To a very large extent, this has been accomplished for severeconductive hearing loss through the development of ossicular replacement prosthesis. (At the age of four, this author was diagnosed with a middle ear disease called a cholesteatoma. Despite low risk of serious damage, the disease developed in both ears, and before the condition was corrected, it had managed to inflict substantial damage to the major structures of the middle ear on both sides.) As many as ten percent of all children develop conductive hearing loss [1], which is hearing loss caused by damage to the ear drum or three bones of the middle ear. (Once the disease had been corrected, this author’s hearing in both ears had been reduced to about 30% of normal hearing.) However, through the use of an implant called a total ossicular replacement prostheses in both ears, these figures were improved substantially to 90% and 60% of normal hearing. Suffice it to say that this device has a major impact on the life of its recipients, as the improvement in hearing one receives is dramatic. The implant that made these improvements possible is widely used to correct conductive hearing loss, and represents a major achievement in biomedical engineering. Those devices used today represent the culmination of over 50 years worth of development, and it is this process which will be the focus of this article.
Background
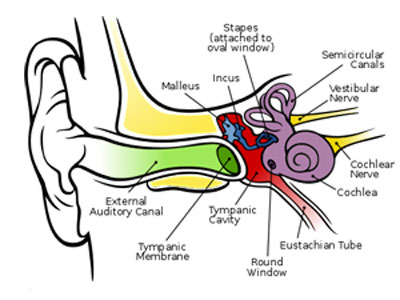
The human ear is typically considered to have three regions: the outer, middle and inner ear. The outer ear serves to collect sound waves; the middle ear translates these signals into mechanical vibrations and conducts them into the inner ear, where the signal is translated into electrical signals to be interpreted by the brain. The middle ear consists of the tympanic membrane (the ear drum) and three ear bones (malleus incus and stapes, known together as the ossicle chain, and is where conductive hearing loss originates [2]). These can be seen in Figure 1.
Conductive hearing loss occurs when any one of the three ear bones becomes dislodged or disconnected, when any one of them becomes bound in place by some inhibiting material, or when the ear bones start to degrade. There are numerous methods through which any of these events could happen. Ear infections, for example, can cause degradation of the bones or the development of scar tissue, which can bind the bones, fixing them in place [2]. Regardless of the cause, any of these defects could seriously affect a person’s hearing.
Partial and total ossicular replacement prostheses (PORPs and TORPs, respectively) were conceptualized with the intent of replacing severely degraded or absent components of the ossicle chain. Most PORPs and TORPs have a relatively simple structure, with some typical designs shown in Figure 2. These devices are surgically implanted; TORPs span between the oval window and the ear drum, and PORPs between the end of an intact portion of the ossicle chain and either the eardrum or oval window. These two configurations can be seen in Figure 3.
The first attempt at replacing the ossicle chain was made during the 1950’s by a pair of German scientists who attempted to use a vinyl acrylic substance to reconnect the base of the malleus to the oval window [3]. This attempt failed because of rejection of the implant, and highlights the main obstacle in the development of these implants: the search for a suitable biocompatible material.. The material must be rigid enough so that it transmits sound well, yet still have a relatively low density to stiffness ratio so that it moves freely with the ear drum. The other major requirement is that the material maintain its properties and integrity for a long time. The material used to create an implant is the most important factor in determining its physical structure, second even to function, although acoustic properties of the material must also be considered.
The Development of Materials for use in Ossicular Implants During the 1960’s in the earliest stage of the development of ossicular replacements, attempts were made to use biological materials such as cartilage or the remnants of the ossicle chain to create ossicular replacements. Such materials were termed autograft materials since they were obtained from the person receiving the treatment. These materials, however, had to be harvested and sculpted during the course of the replacement procedure, prolonging operating time [4]. Another drawback was the possibility that the grafted material had been degraded by the process that had caused the damage to the middle ear in the first place, thereby rendering the grafted material unusable for reconstructive purposes. Homografted materials (biological materials taken from other humans) offered an alternative to autografted materials, which allowed for sculpting prior to surgery.
This cut down on the length of the replacement procedure and eliminated the risk of beginning the surgery only to find that the intended replacement could not be used. Unfortunately, replacements made with these materials demonstrated their own set of drawbacks. This included the risk of resorption and loss of stiffness after 10 to 20 years, as well as a tendency to fuse to the wall of the inner ear. In spite of this, homografted materials were still used with some frequency up until the 1980’s, when they were almost entirely abandoned because of fears regarding the transmission of AIDS through their use [4].
Biological materials clearly had many drawbacks, and so those developing these implants focused on developing synthetic materials to use in ossicular replacements. The first class of such materials was dense polymers, which had a major advantage in that they maintained their stiffness over time, unlike biological materials. As with earlier attempts to use synthetic materials, however, high density polymers provoked negative reactions when in contact with the tissue of the inner ear [3]. Researchers addressed this problem by placing autografted cartilage at the interface between tissue and the implant. However, this greatly destabilized the implant, causing it to dislodge in 3-7% of recipients [3]. The focus turned to developing “bioinert materials,” which were designed so that emissions from the material–which would normally cause negative biologic reactions–would be undetectable. Several of these materials were eventually developed, and these largely replaced high density polymers in implants, since they were accepted much more readily than other materials (Battista). The cartilage interface between the implant and the tissue still proved necessary, however, since bioinert materials would not bind with the tissue of the middle ear without the cartilage interface.
Work in developing superior implant materials was continued until the 1970’s, when bioactive materials were developed. Their name comes from their chemical structure, which promotes chemical adhesion between the tissue and the implant, effectively eliminating the need for a cartilage interface. In particular, hydroxylapatite has stood out among this class of materials. This substance is the main chemical present in human bone, and has been proven to chemically bond with both bone and normal tissue [3]. The only known drawback to hydroxylapatite and other bioactive materials is that they are extremely rigid and brittle. This is not a problem once the implant has been placed, since the rigidity is desirable for acoustical purposes [5]. However, these two factors make the necessary customization of the implant during surgery very difficult. Engineers have attempted to combine this material with surgical-grade rubber, with limited success [3]. Hybrids between bioactive and bioinert materials, which through careful design overcome the weaknesses of both materials, are the end result of years of engineering. This has mostly solved the problem presented by material choice in ossicular prostheses.
Structural Design
Because of the relatively small space into which ossicular implants must fit, and because they must be designed to conduct sound precisely, the general form they have taken has remained relatively consistent throughout the years. This can be seen by comparing modern bioactive/bioinert combination ossicular replacements, seen in Fig. 4, to the earlier bioinert designs, seen in Fig. 2. One of the most important considerations in the structural design of ossicular implants (second only to functionality considerations) has been the need to design them so that they are both easily manufactured and customizable by a surgeon during the implant procedure [5]. The design used to achieve these two goals over the years has varied greatly, since designs have been highly dependent on available materials. Thus, the majority of design innovations have been made in order to accommodate the drawbacks of the available materials. An example of this can be seen in the way that, prior to the development of bioactive materials, it was necessary for the prosthesis to conform to some pre-designed mechanism to allow for the cartilage attachments required wherever the implant was to interface with tissue. This design consideration became unnecessary after bioactive materials were developed, since such materials could bond to tissue directly. The influence of materials on implant designs can further be seen in the design of modern implants, such as those shown in Figure 4. This figure shows a PORP and TORP made of hydroxylapatite. Because of the rigidity of hydroxylapatite, a malleable bioinert-material “neck” has been built into the structure to allow for ease of customization. Further design innovations will be made as new materials are developed.
Future Development
Up to now, designs of TORPs and PORPs have revolved around ease and speed of customization during surgery. This focus has led to a deficiency in knowledge about other areas of possible improvement, such as research into how to more accurately mimic the acoustic response of the ossicular chain with the shape of the prosthesis. Research into this area has been further discouraged by the fact that such a design would require further customization on the part of the surgeon during the operation, prolonging the time required for insertion of the implant [5]. Currently the only acoustical consideration that goes into the PORP or TORP is that they be of approximately the same weight as the original ear bones, since this is of vital importance for a prosthesis to function properly. The creation of prostheses that are both easily customizable and that more accurately model the behavior of the ossicle group represents one of the last unexplored aspects of ossicular replacement design, and will be the focus of ongoing research in order to improve this technology in the future.
The various design aspects of ossicular prostheses have presented their own unique challenges to the engineers involved with the development of these products. The end result of this work has allowed thousands worldwide to lead lives unhindered by a potentially serious disability. For these people, the impact of engineering on their daily lives has been profound, since many everyday activities such as conversation or listening to the radio are made possible for them through ossicular implants. Such implants typically result in substantial improvements in the hearing of those who receive them, although current technology does not allow for the complete restoration of a person’s hearing. Through continued development, it is possible that complete restoration of hearing through the use of ossicular implants might some day become a reality.
For further reading on ossicular replacement prostheses and other hearing improvement devices, visit the House Ear Institute on the web at www.hei.org. For general information on hearing disorders, visit the American Association for Otolaryngology at http://www.entnet.org/healthinfo/.