When you put a spoonful of your favorite ice cream into your mouth, you are enraptured by blasts of flavor, its creamy texture, and coolness on your tongue. Ice cream makers have the same qualities in mind when they are formulating the recipe to their next frozen creation. But, more specifically they are mulling over which ingredients and mixing processes they should utilize to achieve these desired ice cream attributes. Advanced technology and research has allowed us to engineer ice cream in unthinkable ways, ranging from different flavors to controlling the size of fat globules and ice crystals.
A Short History of Ice Cream
The invention of ice cream is not accredited to any individual, but the icy treat’s origin dates back as early as the 12th century in Asia where they used snow to freeze their beverages. According to legend, Macro Polo, the famous 13th century merchant, returned to Italy with “water ice” recipes from the Orient. From here, the popularity of iced water inevitably spread throughout Europe, making its way into high courts and eventually the general population. People began experimenting with different ingredients and freezing processes to meet the public’s desires. For example, as early as 1530, people were freezing water using salt; and by the 18th century, cookbooks started including recipes that used milk, cream, sugar, and eggs, which are common ingredients we see in ice cream today [1, 2].
In the 19th century, the ice cream industry took off in the United States. This was made possible by inventions such as the hand-cranked ice cream freezer, mechanical refrigeration, and the direct expansion ice cream freezer that replaced expensive ice harvesting. At the same time, essential ingredients such as cream, eggs, and sugar became more available and new technologies such as the pasteurizer and homogenizer revolutionized the way ice cream was made [1].
Engineering the Perfect Scoop
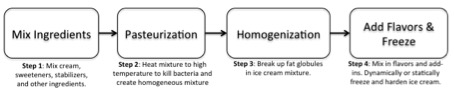
Today, the basic process of making ice cream can be outlined into four steps as seen in Fig. 1: mixing the ingredients, pasteurization, homogenization, and adding flavors and freezing [2]. These processes have been modified to control microscopic (small-scale) and macroscopic (large-scale) properties of the ice cream; for example: flavor, sanitation, melting resistance, and texture.
illumin.usc.edu/illumin.usc.edu
Figure 1: Process of making ice cream starting with mixing the ingredients, pasteurization, homogenization, and ending with the addition of flavors and freezing.
Step 1: The basic ingredients – cream, sweeteners, and stabilizers – of the ice cream are mixed together
When a food chemist or ordinary cook is formulating an ice cream recipe, they must take into account the pros and cons of each ingredient and determine the perfect combinations to achieve the desired properties for the right price. For example, milk contains varying levels of fats that enhance richness and make the ice cream palatable by lubricating and insulating your mouth. On the downside, the cook must consider the high cost and calories of milk-fats. Milk-fats also inhibit whipping, which make the texture more solid and ice-like [1].
When considering sweeteners, cooks often use sugar or corn syrup solids. Sugar causes a freezing point depression, or lowering of the temperature needed for ice cream to freeze and harden. This can result in a softer ice cream product and increases the potential for recrystallization. As shown in Fig. 2, recrystallization occurs when ice cream melts and refreezes, forming larger ice crystals that make ice cream coarse [3]. On the other hand, corn syrup solids cost less than sugar, increase stability, and improve texture. But the cook must be cautious of using too much corn syrup solids, which can result in chewy texture and off flavors.

illumin.usc.edu/illumin.usc.edu
Figure 2: Ice crystals suspended in ice cream.
An additional ingredient that affects the texture and body of ice cream is stabilizers, which are made of large polysaccharide macromolecules. Common stabilizers used include cellulose gum, guam bean, and carrageenan. Hydrophilic (water-loving) polysaccharide stabilizers have the ability to bind with water molecules, which affects the ice cream in a variety of ways. For example: increased mix viscosity, reduced ice recrystallization, resistance to melting, increased product volume and uniformity, and a smooth texture eating experience. Stabilizers ultimately play a crucial role in the shelf life of ice cream, which is determined by ice recrystallization and melting [1].
Although stabilizers enhance multiple qualities of ice cream, an excess of polysaccharides can also result in a chewy texture and off flavor. Additionally, because the benefits of stabilizers are molecular based, different molecules change the way ice cream ingredients interact in a variety of ways. For example, cellulose gum has a high water-holding capacity that contributes to volume; guam bean dissolves readily in cold water and produces high viscosity mixes; and carrageenan interactions prevent phase separation and increase shelf life [1]. Therefore, food chemists must consider different polysaccharide stabilizers to achieve their desired product based on the qualities each stabilizer enhances.
Step 2: The mixture is pasteurized and heated at a high temperature for a short period of time
The process of pasteurization involves heating and mixing the ice cream mixture for a short period of time. Pasteurization is important because the high temperature destroys pathogenic microorganisms, making the ice cream bacteria-free and therefore safe for consumption. Additionally, high-temperature mixing ensures that the mixture is homogeneous (uniform throughout), decreases viscosity (thickness), and denaturizes (unfolds) proteins for increased water retention and volume. Ultimately, pasteurization and the manipulation of the mix components at high temperatures are invaluable for flavor retention, preventing spoilage, and achieving a higher quality product [1, 4]. Without pasteurization, the ice cream mix risks contamination and inconsistencies in the flavor and texture of the frozen product.
Step 3: The hot mixture is passed into the homogenizer under high pressure
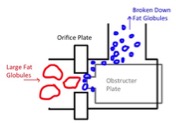
The purpose of homogenization is to modify the fat globules in the ice cream mixture. Fat globules play an important role in the dryness, shape retention, melting resistance, and smoothness of the finished ice cream product [5]. During homogenization, larger fat globules in the ice cream mix are passed through an orifice (smaller opening) in a tube, causing the fat particles to accelerate and collide with a surface, such as the obstructer plate as seen in Fig. 3, and break up into smaller particles. These fat particles interact with each other, play a role in partial coalescence, and form networks that provide structure [5].
Controlling homogenization and the size of fat globules is important for determining the melting rate of and providing structure to the ice cream product. Research has found that there is a negative correlation between fat destabilization (fat globule networks) and melting rate: ice cream with larger fat globule networks had slower melting rates. Scholars such as Hartel, et al. attribute slower melting to an increased flow resistance as the ice crystals melted [6, 7]. Also, if fat globules are too large, excess churning may occur, forming an undesirable cream layer [1].
illumin.usc.edu/illumin.usc.edu
Figure 3: Large fat globules are shot through orifice plate, breaking upon impact on the obstructer plate.
Step 4: The mixture is cooled, aerated, and finally frozen
Flavor is one of the main characteristics of ice cream, and it is during this final step that the flavor is concocted. Food chemists and cooks alike utilize different natural and artificial ingredients to create a wide variety of flavors to fulfill the desires of the consumer. Natural flavors such as vanilla, cocoa, and strawberry are high in demand; but due to limited supply and expenses, flavor chemists work to duplicate these natural flavors by synthesizing and blending compounds to form aromatic chemicals such as vanillin associated with vanilla extract [1]. Additional add-in such as candy bits, caramel, and chocolate chips are also incorporated at this point.
Once the ice cream has finished mixing, special care is taken to freeze the ice cream product. Freezing techniques such as dynamic and static freezing ensure that the desired texture and hardness are obtained. In dynamic freezing, the ice cream mix is quickly frozen and aerated (agitated), which limits the ice crystals’ size, disperses air bubbles, and forms fat globule clumps. Static freezing involves rapid cooling of the ice cream mix and controlled growth of ice crystals in correlation with decrease in temperature [1]. Freezing can take several hours and is dependent on the composition of the ice cream mix that controls the freezing point; a lower freezing point means a longer freezing time [1].

illumin.usc.edu/illumin.usc.edu
Figure 4: The Brrr ice cream maker utilizes liquid nitrogen to flash freeze custom ice cream mixes.
The Future of Ice Cream
Innovations in ice cream technologies today cater to a market that is constantly searching for the newest and coolest scoop. In addition to inventing crazy flavors, ice cream makers have developed new ways to make and present this frozen treat.
Liquid Nitrogen
Since 1987, liquid nitrogen is making its way out of the laboratory and into the kitchen. Microbiologist Curt Jones used liquid nitrogen, which has a boiling point of -320˚F (-195 ˚C), to flash freeze melted ice cream to make what is known today as Dippin’ Dots [9]. Unlike the smooth consistency of traditional ice cream, Dippin’ Dots’ iconic round shapes are formed when drops of ice cream are exposed to liquid nitrogen and instantly freeze [8, 9]. This process is detailed in Fig. 4. Liquid nitrogen macroscopically (on a large scale) alters the structure of the ice cream, resulting in a new ice cream experience without compromising taste.
Liquid nitrogen has also been used to microscopically (on a molecular scale) influence the formation of the perfect ice cream. The extreme low freezing point of liquid nitrogen allows for faster freezing, which results in smaller ice crystals and therefore smoother ice cream. According to studies, the ideal ice crystal size for “smooth” ice cream is less than 50 µm, which can be achieved using liquid nitrogen to flash freeze any ice cream mix [1]. Smitten Ice Cream in San Francisco, California is taking advantage of liquid nitrogen’s ability to rapidly freeze by creating custom-prepared ice cream flavors for customers to enjoy in less than 5 minutes. Smitten uses a machine called the Brrr, which has been designed to effectively mix and create a fresh product without requiring emulsifiers, preservatives, and stabilizers that shelf products require. Using liquid nitrogen, the Brrr is able to consistently make dense, smooth, fresh, and flavorful ice cream creations for consumers to instantly enjoy [10, 11].
3D Printed Ice Cream
MIT engineers Kyle Hounsell, Donghyun Kim, and Kristine Bunker are revolutionizing ice cream production using a soft-serve ice cream machine and 3D printer to make edible works of art [11]. As seen in Fig. 5, 3D printer uses “fused deposition modeling” to print three-dimensional designs one layer at a time. The printer is attached to a soft-serve machine, which is guided by the printer’s software to make the desired design. The set up requires a constant stream of liquid nitrogen to rapidly freeze each layer of ice cream being laid by the soft-serve machine [11]. Using this technology, the ice cream enthusiast has the power to make his or her own ice cream masterpiece at the click of a button.

illumin.usc.edu/illumin.usc.edu
Figure 5: 3D printed ice cream star created by MIT engineers.
Step 5: Enjoy!
The combination of your favorite flavor, creamy texture, and satisfying chill would not be possible without engineering the ingredients and processes that make ice cream what it is today. Making ice cream tackles many challenges including the availability of ingredients, production cost, nutritional value, desired texture and taste, shelf life, presentation, and marketability [12]. In response to changing demands and desires, new technologies and flavors are constantly being developed to satisfy the modern consumers’ ice cream cravings. From the basic ingredients to the spoons of the customers, each step is carefully calculated to create a final product that the consumer enjoys.
References
-
- [1] H. Douglass Goff and Richard W. Hartel, “Mix Processing and Properties,” in Ice Cream, 7th ed. New York: Springer US, 2013, Ch. 5, pp. 121-140.
- [2] BNP Media, “History of Ice Cream. (Chill Out),” in Process Cooling & Equipment, vol. 54, Nov.-Dec. 2001.
- [3] H. Douglass Goff, “65 Years of ice cream science,” International Dairy Journal, Vol. 18, No. 7, pp. 754-758, July 2008.
- [4] M. M. Warren. Understanding melt behavior of ice cream: Influence of the microstructure and composition on drip-through rate of ice cream products. ProQuest Dissertations and Theses pp. 259. 2015. Available: http://search.proquest.com.libproxy2.usc.edu/docview/1736066263?accountid=14749
- [5] H. Douglas Goff, “Formation and stabilization of structure in ice-cream and related products,” Colloid & Interface Science, Vol. 7, Issues 5-6, pp. 432-437, Nov. 2002.
- [6] M. R. Muse and Richard W. Hartel, “Ice cream structural elements that affect melting rate and hardness,” Journal of Dairy Science, vol. 87, no. 1, pp. 1-10, Jan. 2004.
- [7] MMR Koxholt, B. Eisenmann, and J Hinrichs. (2001). Effect of the fat globule sizes on the meltdown of Ice Cream. J Dairy Sci, vol. 84, pp. 35-31.
- [8] R. Pansino, “HOW TO MAKE DIPPIN DOTS WITH LIQUID NITROGEN – NERDY NUMMIES,” [Online Video]. Available: https://www.youtube.com/watch?v=yiXW1SY-NAw
- [9] S. Lohman. (2012, September 2). Origin of a Dish: Liquid Nitrogen Ice Cream [Online]. Available: http://www.fourpoundsflour.com/origin-of-a-dish-liquid-nitrogen-ice-cream/
- [10] Samantha Grossman, “Here’s How Ice Cream Will Look–and Taste–in the Future,” TIME.com, Aug. 2014.
- [11] Smitten Ice Cream. (2016). Who’s Brrr? [Online]. Available: http://smittenicecream.com/story/#slide-476
- [12] D. J. Logie. (2014, February 14). Sweet Secrets From a Ben & Jerry’s Food Scientist and “Flavor Guru” [Online]. Available: http://gizmodo.com/sweet-secrets-from-a-ben-jerrys-food-scientist-and-1522606135




