Aerogel, a material commonly referred to as “frozen” or “solid smoke,” was originally developed in the 1930s, but has not received much attention until now. Scientists and engineers recently realized the possibilities of working with such an unusual substance, focusing on its strength-to-weight ratio and its thermal resistivity. These properties are a result of the material’s molecular structure: it is composed of up to 99% air, with the remainder being a solid polymer network. With hundreds of proposed applications, this substance may be found in everything from building insulation to surfboards to space suits worn by future astronauts on Mars. As with most new technologies, however, the main obstacle to widespread use of aerogel is its relatively high cost.
The Miracle Material
Take a look into the house of the future: sleek, spacious rooms, furniture made from recycled material, and…”frozen smoke”? This frozen smoke, technically known as aerogel, is an extraordinary material that may find a way into your home sometime in the near future. As engineers discover ways to make this substance more affordable, the number of practical applications is increasing at an amazing pace. From home insulation and blankets to tennis rackets, ski jackets, and refrigerators, aerogel may revolutionize many facets of everyday life.
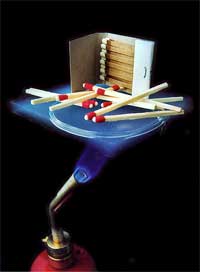
So what exactly is aerogel? While some materials may exhibit a low density, high thermal resistivity, or high strength-to-weight ratio, only aerogel can claim all of these properties at once [1]. The name aerogel comes from the combination of the Greek word “aero”, meaning air, and “gel”, since aerogels are derived from gels. Normally, a gel is approximately 99% liquid, while the remaining 1% is a solid network that spans throughout the liquid and can allow the gel to maintain its shape. With a density only three times that of air, aerogel is what is left after replacing the liquid contents of a gel with a gas — without shrinking it [2]. As shown in Fig. 1, the result is an opaque blue solid that is composed of anywhere from 95-99% air. The texture of aerogel is similar to a fine, dry sponge, but feels much lighter [1]. In fact, aerogel holds the record as the lightest solid in the world. When pressed softly, aerogel will return to its original form, but when pressed harder, a dimple forms. Put aerogel under too much pressure, however, and it will shatter like glass into many tiny pieces. Although it was first produced over 70 years ago, it has taken recent research and applications for it to be hailed as a “miracle material” [3].
It Started With a Bet
In the early 1930’s, Stanford University’s Samuel Kistler had a bet with a fellow scientist over who could first remove the liquid portion of a gel without compromising its volume. Kistler won, and aerogel was the result. Normally when a gel dries, it shrinks to a fraction of its original size because the surface tension of the liquid leaving the gel collapses its polymer structure. Working with the knowledge that a liquid at sufficiently high temperature will become a gas, Kistler developed the process of supercritical drying, or raising the temperature and pressure on the gel so that it reaches the point where the liquid in the gel is no longer distinguishable as liquid or gas. Once in this supercritical state, the molecules of the liquid can be removed and the remainder slowly cooled to form aerogel [4]. At the time, aerogel did not receive much recognition and was not considered for any contemporary applications because of high costs and its lengthy and volatile method of production.
Aerogels would not make another major appearance in the scientific world until the 1980’s, when a group of researchers led by Arlon Hunt of Lawrence Berkeley Laboratory came upon a sample [5]. Hunt and his team worked to improve the material’s clarity, production methods, and thermodynamic properties. One of the first projects the team worked on was finding a safer way to produce the substance. The base chemical used to make aerogels, tetramethylorthosilicate (TMOS), is highly toxic and can easily damage the human eye. At the time, it was known that aerogels could be made using a safer compound, tetraethylorthosilicate (TEOS), as a substitute for TMOS, but the resulting aerogel was smaller and less clear. Hunt’s team experimented with the other chemicals involved in aerogel production and was able to achieve a clearer and less shrunken TEOS aerogel.
Although this accomplishment made aerogel production slightly safer, it was still not safe enough to attain widespread use. Hunt continued to work toward improving aerogel production after hearing news that an aerogel lab in Sweden had exploded. Originally, aerogel’s drying process required alcohol to be exposed to extremely high pressures and temperatures, creating a very dangerous, combustible environment. Hunt found that liquid carbon dioxide was an excellent substitute for alcohol, not only because it was inflammable, but also because it required less heat and pressure to become supercritical (see Fig. 2). Therefore, using liquid carbon dioxide not only made the process safer but also more energy efficient and cheap enough to be considered for commercial manufacturing [5].
One Substance, Many Uses
Thanks to Hunt’s innovations, aerogel has recently been engineered for use in many applications, notably by NASA to catch tiny space particles left behind by comets. In 2006, NASA’s Stardust spacecraft successfully returned with these interstellar dust samples. These particles usually disintegrate upon impact with a solid or liquid. But due to aerogel’s fine microstructure and the fact that it is mostly composed of air, the particles were able to be caught intact in an aerogel “mitt.” The particles break through the aerogel’s polymer chains and gradually come to a stop, while remaining intact [1].
Aerogel has also found its way into today’s clothing. The engineers of one aerogel company, Aspen Aerogels, have developed aerogel “blankets”, flexible linings that can be used in snow outerwear. Since the molecules in aerogel are only weakly connected to each other, they are very poor conductors of heat [4]. This makes aerogel ideal for clothing insulation since it is much better at resisting heat transfer — at a fraction of the weight and volume of normal insulation. In one case study, aerogel insulated sleeping bags and boot insoles were used by a female mountaineer to summit Mount Everest. In the sub-zero temperatures, she claimed that the only problem she had with the insoles was that her feet were too hot! The Hugo Boss fashion company encountered the same problem with its line of aerogel-insulated winter jackets. The company eventually had to pull the line because of complaints that the jackets were too hot to be worn comfortably [3].
Sporting goods companies are also beginning to use aerogel in their products. Aerogel’s remarkable strength-to-weight ratio has convinced English sporting goods manufacturer Dunlop to release a line of aerogel-reinforced tennis and squash rackets. The company’s promotion of the line claims that their latest technology is the next generation of rackets. Burton Snowboards has also been developing a line of aerogel-insulated outerwear.
The Future
Aerogel is expected to make a significant impact in sustainable and “green” development. Because of aerogel’s exceptional thermodynamic properties, it is not just an ideal insulator for clothing, but for buildings as well. Aerogel wall insulation can achieve the same insulation effectiveness as traditional fiberglass at less than a third of the thickness [6]. In addition, aerogel can be made hydrophobic, allowing it to prevent mold growth by repelling water vapor. It is also possible that aerogel may replace today’s windows. One of the main challenges facing engineers is making the material more transparent. Once the blue tint is removed, aerogel can be put between double-pane windows to further limit heat transfer in buildings. According to NASA, current opaque aerogel has the same insulating ability as 10-20 glass panes [7]. Fig. 3 demonstrates aerogel’s unparalleled ability to resist heat transfer.
Other predicted uses of aerogel include armor for military vehicles and a “sponge” utilizing aerogels’ pores to soak up lead and mercury spills in the ocean. The idea of aerogel armor came after experiments showed a metal plate with a protective aerogel layer was found to sustain little damage when exposed to a blast of dynamite. Also, Aspen Aerogels has taken their clothing insulation a step further, and plan to fabricate a space suit for a manned mission to Mars in 10 years. Scientists at the company believe that the insulation will be able to withstand temperatures as low as -130 C. There may be another “green” use of aerogels as well, as research has found that platinum-based aerogel can speed up the production of hydrogen for use as fuel [3]. If implemented successfully, this technology would be able to provide cheaper alternative fuels to combat the high cost and limited supply of oil.
With most aerogel applications, however, cost is still a prohibitive factor. For now, although aerogel insulation is much more effective at retaining heat compared to typical fiberglass insulation, fiberglass insulation is unlikely to be replaced, mostly due to its low cost [8]. Recently, Aspen Aerogels announced plans to increase their plant size to produce larger quantities of their aerogel blankets and in turn reduce their cost. It seems, though, that aerogel production needs just a few more engineering breakthroughs to finally become affordable enough for use widespread use. If the last 20 years are any indication, it is probably just a matter of time before we are surrounded by this remarkable material.