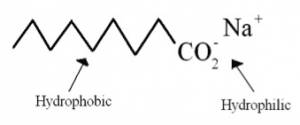When people kneel down to scrub the stains out of their bathtubs or struggle to remove the coffee grounds from their kitchen counters, they are probably not thinking about the complicated chemical reactions that are happening just beneath their hands. The cleaning supplies that we all use, from simple soaps to detergents to vinegars and bleaches, have all been carefully engineered and designed to break down various types of soil and keep our homes as clean and safe as possible.
Introduction
Have you sanitized your sponge today? Dr. Philip Tierno, the Director of Clinical Microbiology and Immunology at New York University Medical Center, found that the greatest amount of infectious bacteria in the home comes from dirty kitchen sponges [1]. Moreover, rotavirus, a virus causing severe diarrhea, contaminates remote controls, light switches, and door knobs [2]. Shockingly, Tierno’s research also showed that fecal matter contaminates toothbrushes because toilets spray as much as 20 feet when flushed [1]. Cleaning these contaminants represents an essential part of our daily lives and allows us to live in a healthy environment.
The first step in deciding which cleaning agent to use is figuring out the category of the soil or dirt we want to remove. Once this is determined, options range from the first widely used cleaning agent, soap, to modern chemical creations, such as detergent, and finally to common household products, such as vinegar and bleach. The various chemical structures and properties of each of these agents will determine which is most appropriate for each situation.
For our purposes, “soil” is defined as matter that makes a surface dirty or that is in an undesired place. For instance, pizza is a delicious food, but when it is rubbed into the carpet it becomes soil that needs to be removed [3]. Soil can be grouped into three categories: organic, inorganic, and a mixture of the two [4]. Organic soils are made up of two main groups: food and petroleum. Food soils include grease, fat, mold, and bacteria, while petroleum soils include motor oil and axle grease. Inorganic soils include hard water, rust, scale, minerals, sand, and clay [3]. Mixtures or a combination of organic and inorganic soil, like the ever-elusive soap scum, pose the greatest removal challenge for any cleaner. These soils require an ideal mixture of organic and inorganic cleaning agents.
Soap
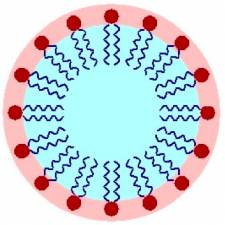
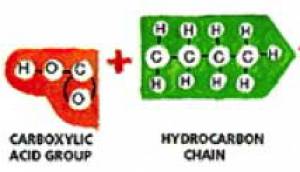
Soaps, the most common cleaning agents, remove organic soils. Basic soaps are composed of water-soluble potassium fatty acid salts (see Fig. 2) [5]. They are made from fats and oils that are treated strongly with an alkaline solution [6]. In soap, three fatty acid molecules are attached to a glycerin molecule (see Fig. 2). The molecules in soap have two important components: the head and the tail. The head of the soap molecule is a negatively charged carboxylate anion (CO2-), which is hydrophilic, or “water loving.” The tail of the soap molecule has a long hydrophobic, or “water hating,” hydrocarbon chain (see Fig. 1). In water, the hydrophobic tails congregate together, while the hydrophilic ends attract other water molecules to result in the formation of a micelle structure (see Fig. 3).
In the presence of soil, the hydrocarbon tail attaches to the organic soil as the polar carboxylate “head” pulls the dirt off the surface. Unfortunately, traditional soaps also react with minerals in water to produce soap scum, a combination of organic and inorganic soils that is difficult to remove.
Detergent
Although soaps have frequently been used to clean most organic soils, modern engineers have manufactured more potent cleaning agents, known as detergents. Over the years, detergents have been engineered to enhance cleaning power while leaving less soap scum, the common drawback of regular soaps. Detergents are widely used to clean dirty dishes and soiled laundry. General detergents are composed of four main parts: surface active agents or surfactants, chelating agents, builders, and solvent.
Surfactants
Surfactants help clean organic solvents from surfaces [7]. Similar to soap molecules, these chemicals have two key components.
One part of the molecule has a long non-polar chain, known as the hydrophobic end, which repels water. The other part of the molecule is the polar, or hydrophilic, end which attracts water [3].
When these surfactants are in a solution containing soil, the hydrophobic end becomes attracted to the organic soil (oil, grease, etc.). The hydrophobic end surrounds the soil and the hydrophilic end pulls it into solution (see Fig. 4). Eventually, the surfactant removes all the soil from the surface and suspends the dirt in the solution, which can then be washed away (see Fig. 3) [4].
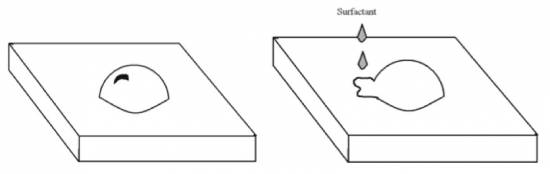
Surfactants aid in the cleaning process because they make water “wetter” [4]. Water has high surface tension, inhibiting the cleaning process by not allowing water to spread and wet a surface. High surface tension in water is due to hydrogen bonding, a strong intermolecular electronic force. The positive and negative ends of the water molecules attract each other by acting like little magnets to pull water molecules together (see Fig. 4). Just think of what happens when you place a drop of water on a table – it forms a small bead due to the water’s surface tension and doesn’t spread (see Fig. 5). Surfactants reduce water’s surface tension and allow it to spread easily and cover the surface, thus aiding in the cleaning process (see Fig. 5) (Soaps, Detergents and Cleaning).
The chemical process of cleaning is complicated because water generally contains dissolved metal ions like calcium, magnesium, iron, and manganese. These metal ions disturb the surfactant cleaning process by reacting with the surfactant exactly like soil does. This means the surfactant is used up by the metals, without cleaning the soils [3]. To alleviate this problem, chelating agents are added to surfactants. Chelating agents work by the same fundamental forces that cause surface tension and intermolecular electronic attractions. These chemicals have many negatively charged atoms bonded to them. When the chelating agent comes into contact with a positively charged metallic atom, electronic forces cause them to bind together. Through this process, negatively charged chelating agents and positively charged metals react to prevent unnecessary loss of the surfactant (see Fig. 6) [8]. Common chelating agents include EDTA (ethylene diamine tetra acetate) and phosphates.
Builders
A multifunctional alternative to chelating agents are builders, which help to reduce the hardness of the water, buffer the solution, and emulsify the soil [3]. Water is considered hard when it has a high concentration of mineral ions such as calcium and magnesium dissolved in it. Metal ions such as iron, aluminum, and manganese also contribute to water hardness. There are two methods by which builders reduce the hardness of the water: sequestering and precipitation [4]. The sequestering method softens the water by attracting the metal ions in solution, exactly like chelating agents. The precipitation method works by reacting special chemical builders with metal ions. The reaction causes them to be insoluble in solution, meaning they can no longer dissolve in water. Builders are often used instead of chelating agents because they are cheaper.
Builders also increase the pH, thereby buffering the solution and aiding in the cleaning. The buffer charges the soil, consequently allowing the charged water molecules to more easily remove it. A builder’s third purpose is to emulsify the soil, helping to break up oil and grease into smaller fragments and suspend the loose soil. In addition, they prevent the loose soil from settling back on to the surface. Phosphates (tripoliphospate), sodium carbonate (soda ash), and sodium silicate are often used as builders [3].
Solvents
Surprisingly, in most household liquid cleaning agents, water makes up more than 50% of the liquid. In some water-based detergents, water makes up close to 95% of the solution [4]. In fact, water is one of the most important components of detergents because it increases the strength of the cleaning agent. In a detergent, water performs several important tasks. Water is the solvent that dissolves the soil once the surfactant lifts the dirt from the surface. Solvents help to ensure that once soil is removed from the surface and emulsified, it does not get re-deposited onto the now clean surface. Solvents keep the dirt suspended and away from the surface so it can be easily rinsed away [4]. Imagine trying to clean a kitchen counter without water; it would be difficult to spread the thick detergent, and even more difficult to wash it away.
Vinegar is the perfect cleaning agent to remove both inorganic and tough combination soils, and it can replace many expensive commercial cleaners. Consumers often buy expensive cleaners to keep their toilet bowls sparkling and their homes spotless, despite the fact that these conventional household cleaners are some of the most toxic substances one can bring into the home. Many have not been tested for long-term health effects [9]. Additionally, possible health risks from cleaners include asthma attacks, headaches, dizziness, memory impairment, and visual disorders, according to the Environmental Protection Agency [9]. Household vinegar is a nontoxic alternative to conventional cleaners with many uses in the home.
Vinegar has an acidity of 2.5 to 4.0 on the pH scale [10]. The acidity of vinegar helps to dissolve soil particles by making them charged. These newly charged soil particles become attracted to the positive and negative charges in water. The soil is pulled into the water by strong intermolecular electrostatic forces and thus can be easily removed [10]. Vinegar is extremely helpful in places with shower head corrosion, toilet bowl stains, soap scum build-up, and various other mineral surface deposits. Vinegar can also be used to clean up the dark green and black inorganic coating that develops on copper and brass metals, which are often used for cooking utensils and jewelry [8]. Copper and brass tarnish because the metallic elements react with carbon dioxide and sulfur dioxide in the air. These deposits can be removed because they easily become charged by the acidity of vinegar [8].
Bleach
Bleach, a light yellow-green transparent liquid, is used to remove unwanted color and stains through an oxidation reaction process. There are several kinds of bleach, but the most widespread form is chlorine bleach, chemically known as sodium hypochlorite [11]. Bleach eliminates stains because it breaks apart the bonds of the chromophores in molecules. Chromophores are the parts of molecules responsible for color. Through an oxidation reaction that breaks these bonds, bleaching makes the compounds colorless [6]. Therefore, the stain can no longer be seen. A reducing bleach works by changing the double bond in chromophores to a single bond, once again making the compounds that cause a stain colorless [12].
The chlorine in bleach also helps kill bacteria because it disrupts their biological processes. When chlorine bleach is added to water, it forms many different chemicals, one example being hypochlorous acid (HOCl). This chemical and others work to kill microorganisms and bacteria. Due to its small size, the hypochlorite anion (ClO-) kills bacteria by diffusing through the bacteria’s cell wall and disrupting its ability to function [13]. The compound attacks lipids in the cell wall, causing destruction of enzymes and other parts of the cell, leaving the bacteria harmless [14]. Although bleach can be a useful disinfectant and stain remover, it is a dangerous chemical that can inflict harm to human cells the same way it attacks unwanted bacteria.
After Hurricanes Katrina and Rita, many affected residents wanted to remove organic bacterial mold from their homes and disinfect them. According to the Louisiana Department of Health and Hospitals, bleach was mixed with other cleaning agents, which unknowingly produced toxic substances. Poison Control explained that mixing the substances led to skin irritations and respiratory failure. Generally, two chemical cleaning agents should never be mixed. They may be innocuous when separate but deadly when mixed together.
Conclusion
Despite decades of engineering research to discover more effective cleaning agents, current cleansers still have notable limitations, with soaps producing bathtub soap scum and bleach remaining an extremely hazardous chemical. People often do not recognize that even common cleaners are chemicals. These chemicals cleaners can be extremely hazardous if they are used improperly. Therefore, chemical engineers are left with the challenge of creating a versatile cleaner that is effective in removing many types of soil, environmentally friendly, and harmless to humans.