Kari Hernandez was a junior majoring in Industrial and Systems Engineering, with emphasis in Information Systems and Operations Management at the time she was published. As a young woman, she is familiar with current birth control methods, but has an interest in the newly conceived male counterpart.
The cross-cultural desire for a male birth control “pill” has led the drive for a drug that creates reversible infertility in a safe and reliable manner, while securing sexual freedom and allowing men to partake in the responsibility of family planning. Potential drugs being explored are either hormonally-based and impede the production of sperm, or they are non-hormonal and render the sperm dysfunctional. Both methods have seen promising results; the former having potential correctible short-term side effects. Researchers aim to develop a drug that avoids major health-related issues and a decline in sexual drive, ensuring public interest in a male contraceptive. Long-term side effects for both forms are largely unseen. Although estimated to be about 10-15 years from production and prescription, such a birth control process has already identified potential markets worldwide, especially in Asia.
Introduction
Each year, 3 million unplanned pregnancies occur in the United States alone. On average, 468,988 teenagers will give birth, and 78% of these are a result of unplanned pregnancies (www.americanpregnancy.org). Despite all of the available male birth control methods, female birth control, namely the “pill,” is still the most effective means of preventing pregnancy. Around the world, leading researchers are currently exploring the development of a male contraceptive “pill.” The goal: to create a safe, reversible, accessible male contraceptive with few, if any, negative side effects. Researchers are currently investigating not only the desire for such a drug, but also numerous possibilities for its development. So far, developmental efforts have focused in two realms: hormonal and non-hormonal drugs.
The hormonal solution, as its name implies, uses various hormones to stop or significantly reduce spermatogenesis and has been proven successful in many different studies worldwide. Essentially, the hormonal contraceptive inhibits the entire male reproductive system, more specifically primary male sexual characteristics. The non-hormonal counterpart, on the other hand, focuses not on limiting sperm production, but rendering sperm unable to swim or successfully fertilize an egg. Preliminary results from the non-hormonal drug tests show fewer adverse side effects than in the hormonal variant; however it is much further from completion.
The difficulty in designing a male contraceptive lies in the struggle to control the millions of sperm involved in fertilization rather than the one egg females release on a monthly cycle. Despite this difficulty, the research efforts have proven largely successful in producing reversible infertility in both rodent lab tests and human field studies. The benefits of developing such a drug and confirming markets encourage researchers and pharmaceutical companies alike to pursue and promote a final, marketable, mass-producible product that will significantly alter society’s notions about birth control.
Overview of the Male Reproductive System
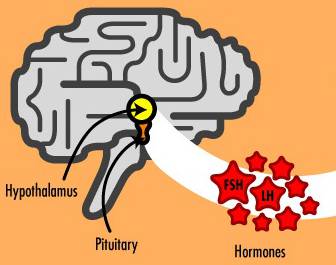
In the male reproductive system, the hypothalamus produces gonadotropin-releasing hormone (GnRH), which in turn triggers the pituitary gland to secrete follicle-stimulating hormone (FSH) and luteinizing hormone (LH) [1]. These hormones are essential in triggering many actions of the reproductive cycle. LH then causes the testes to produce testosterone, a steroid hormone that impacts sexual drive and the development of secondary sex characteristics such as facial hair and muscle mass. In addition, high levels of testosterone are responsible for the primary male sex characteristic: production of sperm in the testes (see Fig. 1). Millions of sperm are produced each day, each with a maturation period of about 75 days [2].
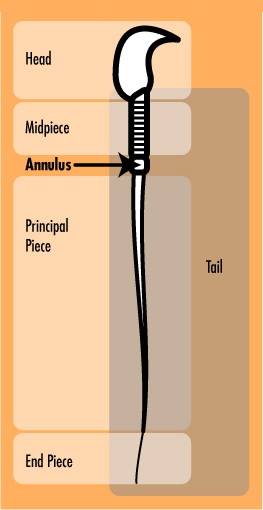
Upon sexual stimulation, a man climaxes with ejaculation – the expulsion of up to 500 million sperm. Sperm are generally divided into two basic units: a flagellum and head. The flagellum enables the sperm to swim, while the head carries genetic material that is passed along to the egg during fertilization.
Calcium channels play an integral role in sperm functionality both for swimming and fertilization. These channels, which are actually composed of proteins, function much like a door: each channel protein has an active site requiring a specific activating agent to bind to it before the channel will open. In fact, these activating agents often will cause the proteins to change shape entirely. One such integral protein is CatSper, discovered in 2001 [3]. Activated by alkaline conditions, CatSper permits the passage of calcium into the sperm, initiating a mechanism that allows the sperm to swim. In addition, calcium channels on the head of sperm, have proven essential to the penetration of the sperm head through the corona during fertilization [3].
The tail of a sperm is further divided into three subunits: the midpiece, principal piece, and the end piece (see Fig. 2). The annulus, composed of septin proteins, separates the midpiece and principal piece of the tail and is considered essential for proper sperm function. Scientists of Population Council, a nonprofit, international organization researching human reproduction, hypothesize that the function of the annulus is to maintain a physiological balance between the chemical environments of the midpiece and the principal piece, ensuring the physical structure and strength of the sperm tail [3]. Without the annulus, the tail would not be as straight and rigid as it should be and would function improperly.
How a Male Contraceptive Would Work
The male contraceptive “pill” will most likely not be a pill at all, but rather an injection, patch, implant, or combination of the three. The major aversion to develop an ingested contraceptive draws from the potentially serious side effect of liver damage from excessive hormone concentrations in the digestive tract [4].
Developing a GnRH Inhibitor
Steroid hormones called androgens control secondary sexual characteristics in humans. As mentioned earlier responsibility of the androgen testosterone extends further, into primary sex characteristics. To restrict, limit, or terminate the production of sperm would require controlling the testosterone production in the male reproductive system: the precise goal of a hormonal male contraceptive.
The first testosterone-regulating experiment was accomplished through an orally administered synthetic androgen. The trick to such an approach is that the synthetic hormone appears to the human body as testosterone, but does not function; the hormone version of the placebo test. Therefore, the body senses appropriate hormone levels and does not produce gonadotrophins, but since the pseudo-testosterone is dysfunctional, the body does not produce sperm. Although successful, this method was quite successful, the testosterone-based drug actually had a negative effect on the liver and prostate gland [2]. Increased levels of the pseudo-testosterone proved to enlarge the prostate, an early indicator of susceptibility to prostate cancer.
After experimenting with various testosterone-derived hormones and administration methods, the Population Council stumbled upon one of its own synthetic androgens, MENT (7-alpha-methyl-19-nortestosterone). MENT was originally developed as a breast cancer treatment that suppressed the production of estrogen, but was soon observed to boast ten times the potency of testosterone without enlarging the prostate gland [2].
Unlike other testosterone based treatments, the MENT based contraceptive functions much the same way as the female birth control pill. MENT stimulates the male immune system to produce antibodies that inactivate GnRH, effectively inhibiting sperm production [5]. Moreover, since MENT is not a dysfunctional version of testosterone, and is quite potent, it helps maintain secondary male sex characteristics. In fact, MENT also has the potential to be used as a supplement for testosterone deficiency [5].
Implants have proven to be the most favorable method of application among leading researchers. One, two, or four MENT Acetate implants may be placed under the skin of the upper arm, and have shown to be successful fertility inhibitors for up to a year after initial application [6]. In addition researchers have sought the use of a transdermal patch (like the nicotine patch). Curiously, it has been determined that combining the synthetic androgen (MENT) with a progestin, a hormone used in female birth control, increases the efficiency of the drug when used in males.
Unfortunately, the side effects of MENT are largely unknown. Common short-term side effects include acne and weight gain; however the weight gain observed is not fat tissue as seen in female patients using birth control. The growth actually occurs in muscle tissue, similar to the way athletes using steroids increase muscle mass. Long-term side effects are completely unknown at this point, and are currently being investigated. Researchers will try to determine if there is the potential for immune system danger and tissue damage after extended use of the drug [7].
Creating Spermatic Dysfunction
In order to successfully swim and fertilize an egg, two elements must be present in a sperm cell: an annulus and calcium channels. Development of a non-hormonal drug to combat male fertility aims specifically to target one or both of these mechanisms.
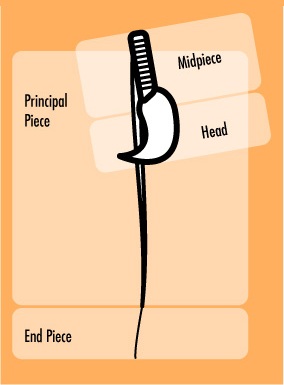
So far, two proteins have been the primary targets of researchers: Septin 4 and CatSper [3]. In mice lacking the gene for Septin 4, sperm cells formed without an annulus. Without the annulus, the sperm cannot swim correctly because its tail is actually bent backward at the point where the annulus should have been (see Fig. 3). Moreover, without the annulus, the sperm head actually cannot separate from the tail, making it unable to fertilize an egg.
In blocking CatSper, the crucial calcium channel would not function properly, allowing the sperm to neither swim nor fertilize an egg [8]. Scientists are especially optimistic about this approach because selective calcium channel antagonists have been designed for health conditions including high blood pressure, migraines and Raynaud’s disease.
Will Male Contraceptives Have a Market?
Carl Djerassi, “father of the birth control pill,” says that while it may be possible to engineer a male birth control method, pharmaceutical companies will be too reluctant to produce it [9]. In his opinion, the threat of even a slight chance of impotence or permanent infertility would be enough to frighten away the majority of prospective clients.
Alternatively, the Population Council believes that the economic opportunity is endless, pending continued studies and development. The organization believes that a male contraceptive will first find success primarily with men in monogamous relationships. Men worldwide, including Australians, Chileans, Europeans and Asians, have volunteered themselves for ongoing research. Researchers confidently believe the biggest initial market will be in Asia [4]. Estimates for the completion of a viable male contraceptive range between ten and fifteen years.
It is important to remember that some unfavorable circumstances may arise from the successful completion of this project, such as the potential of a significant increase in transmission of STIs and STDs. However, these dangers can be mitigated by a heightened awareness, considering that the risk of pregnancy will be taken out of the equation. The development and mass production of such a drug could even reduce unplanned and teen pregnancy. With such protection, the world could expect an overall healthier family environment, a decrease in poverty levels, and a slowdown in overpopulation rates. It appears the “man pill” is a much more than a potential birth control method, it has the potential to become a major social driving force.
References
-
- [1] Male Hormones. Andrology Australia, 2001. Internet: http://www.andrologyaustralia.org/malebody/malehormones.htm. Accessed: 29 March 2006.
- [2] L. Berger. Quest for Male “Pill” Is Gaining Momentum. The New York Times. 10
- [3] G. R. Hunnicutt et al. (2003, Jan.). “Studies on the Function of the Annulus in the Sperm Tail”. Population Council Research. [On-line]. Available: http://www.popcouncil.org/projects/BIO_AnnulusFunction.html [March 5, 2006].
- [4] J. E. Altwein et al. (2005, Apr). “Reversible, Non-Barrier Male Contraception:
- Status and Prospects”. Europeanurology. [On-line]. Available: http://europeanurology.com/article/S0302-2838(05)00144-2/pdf/Reversible,+Non-Barrier+Male+Contraception%3A+Status+and+Prospects.
- [5] K. Best. “Contraceptive Update: Experimental Male Methods Inhibit Sperm”. Family Health International, Network: Spring 1998, vol. 18, no. 3.
- [6] UNIVERSITY OF EDINBURGH. (2006, Feb.). “Etonogestrel + decanoate has potential as a long acting male hormonal contraceptive”. Biotech Law Weekly. [On-line]. Available: http://web.lexisnexis.com/universe/document?_m=3a7542ab70abe7c0d6c7a434b757523b&_docnum=4&wchp=dGLbVlb-zSkVA&_md5=9d28854ea64a703fc648 8a9c41ea4c3e. [Mar. 7, 2006].
- [7] G. K. McEvoy. “AHFS DRUG INFORMATION(R) 10:00 Antineoplastic Agents, Leuprolide Acetate”. American Society of Health-System Pharmacists, Inc., 2006. Internet: //online.statref.com/document.aspx? fxid=1&docid=266, 2006 [8 March 2006].
- [8] (2006, Mar.). “Experiments Pin Down Target for Male Contraceptive”. Life Science Weekly. [On-line]. Available: http://web.lexis-nexis.com/universe/document?_m=3a7542ab70abe7c0d 6c7a434b757523b&_docnum=17&wchp=dGLbVlbzSkVA&_md5=ad6d2dae4a7873e4e 9f13f840b94b43f [Mar. 8, 2006].
- [9] “Father of birth control pill says male version possible, unlikely”. Agence France Presse. Lisbon.
- [10] (2002, Dec.). “Contraception: Status and Prospects”. European Urology. [On-line]. Volume 48, Issue 5, pp. 712-723, via Science Direct.
- [11] Internet: http://query.nytimes.com/gst/fullpage.html?sec=health&res=9F06E4DD163AF933A25751C1A9649C8B63 [4 March 2006].