Zenzile Brooks was a junior at the University of Southern California (USC) in the spring of 2005. Her extra-curricular activities include serving as Vice President of the USC Chapter of the National Society of Black Engineers (NSBE), tutoring as a Supplemental Instruction Leader for Math126 (Calculus II), running, reading, and reorganizing her refrigerator.
The refrigerator is an important instrument of food preservation for modern society. The refrigeration cycle is the chemical process that drives the refrigerator and generally consists of four main steps: 1) compression of ammonia refrigerant; 2) cooling of ammonia refrigerant; 3) expansion of ammonia refrigerant; and 4) drawing in of heat from the core of the refrigerator. Many variations on this process exist as manufacturers find new ways to improve the temperature-control aspects of the refrigerator.
Introduction
Chances are, he’s in your house right now. He’s big. He’s cold. And he knows where you keep your food. As a matter of fact, he is where you keep your food. He’s your refrigerator. And he’s in for a surprise.
Refrigerators have been a hallmark of American family life in recent decades. Today, a century after the refrigeration cycle revolutionized the concept of food storage, the refrigerator is gearing up for a makeover. Modern science and engineering principles are combining in the latest refrigerators, and they’re on their way to a kitchen near you in the not-too-distant future.
From the Cave to the Kelvinator
Although it is nearly impossible to fathom now, modern refrigeration was not a part of daily family life until the 19th century. Prior to today’s beloved, compact steel boxes, people stored food in the coldest places they could find or make. Ancient Greeks, Romans, and Hebrews covered the food they wanted to keep cold with snow, and topped it off with an insulating material to keep the heat out and the cold in. The Chinese harvested and stored ice before even the first millennium [1]. Many other pre-industrial societies stored food below ground, in cellars and wells [2].
The pioneers’ rudimentary but effective cold spaces incorporated the most important part of the modern refrigerator: the refrigerant, the substance that circulates through the refrigerator to make the food cold. For these early, underground “refrigerators,” the refrigerant was water. The “refrigerator” would be a small room or chamber with water trickling down the walls by means of small holes. Pioneers also employed a variation on this technique by building a “springhouse”, a small house over a spring. They placed buckets of cream and butter into the naturally running water to keep their content cold [2]. Unfortunately, these methods were not without their shortcomings; food often spoiled anyway and many unfortunate pioneers fell victim to “summer complaint,” a sickness obtained from the bacteria of spoiled food [1].
In the mid 19th century, Frenchman Ferdinand Carre devised a more recognizeable form of the modern refrigerator. Carre began experimenting with the concept of circulating a coolant around a core (Ideafinder). In his “vapor-compression refrigeration system,” an instrument called a compressor circulated ammonia around the cool core of the system. Carre’s system became popular, but ultimately proved too large, expensive, and dangerous to become a real household item. Many continued to rely on iceboxes [3].
Raoul Pictet of Switzerland and Karl von Linde of Germany both modified Carre’s design to produce more practical refrigerators. The first domestic refrigerator entered the scene in 1913 thanks to Chicago’s Fred W. Wolf, Jr. He coined his machine the “Domelre,” short for Domestic Electric Refrigerator. Wolf’s invention met with little success. The subsequent Kelvinator was more widely sold, but General Electric’s 1927 “Monitor Top” refrigerator won the early refrigerator popularity contest. The company produced over 1,000,000 units, some of which still function today [2].
The Star of the Show
Step inside your kitchen, take hold of your refrigerator, and spin it around. On the back, you will find the three key elements of the modern refrigerator: the compressor, the expansion valve, and the heat-exchanging pipes. These pieces work together to push, pull, and pump the lifeblood of the refrigerator, the refrigerant- ammonia in today’s refrigerators- around the fridge.
The circulation of the refrigerant was crucial to even the earliest of refrigerators. The biggest difference between today’s Maytag and the pioneer’s hole in the ground is that the modern refrigerator controls the circulation of its refrigerant, or cooling substance. Whereas the wells and springhouses discussed earlier relied on the continuous entry of fresh, cold water, today’s refrigerator pumps the same refrigerant around and around. Ammonia is a very special substance and an ideal refrigerant because of one crucial quality: its low boiling point. Ammonia starts to boil at very low temperatures.
In other words, place ammonia in the vicinity of a room temperature ketchup bottle and it will complain that the ketchup is too hot. Refrigerants like things cold. The weak bonds between their molecules can’t handle even a little bit of heat, and are likely to transfer it to some external source.
When ammonia gets near anything room temperature or warmer, the heat breaks ammonia’s delicate bonds and it boils. The same thing happens with a pot of water on the stove; once the stove gets hot enough, the not-so-delicate bonds between water molecules break and the water boils. Ammonia boils at an ideal temperature for food preservation:-28 degrees F (Ammonia).
If the refrigerator was a person, the refrigerant would be the blood. Refrigerant flows through the casing of the refrigerator and absorbs heat to make the food cold. When the ammonia gets near the room temperature ketchup bottle inside the fridge, the heat from the bottle leaves the bottle to boil the ammonia, and the bottle gets cold.
The Refrigeration Cycle: Preparing the Ammonia
The refrigerant is the most crucial component of the refrigerator. In modern refrigerators, the refrigerant follows a simple process to keep food cold. The first two parts, the compressor and heat-exchanger, bring the ammonia to the best physical state for performing its duty of stealing heat. The next two parts, the expansion valve and coils, carry the ammonia near the food so it can steal heat and keep the food cold.
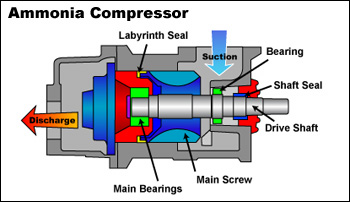
The ammonia circulates throughout the refrigerator continuously, but we’ll pick the compressor as our starting point. This is the first crucial instrument that helps prepare the ammonia. The compressor heats the ammonia by compressing it. The ammonia enters the compressor through the blue arrow at the top of the diagram. Suction drives the ammonia molecules through the compressor from this large opening to the smaller opening of the discharge tube.
Despite all the bells and whistles, the compressor operates on the simple principle of pushing the ammonia molecules together. From the ammonia’s perspective, the exit is smaller than the entrance. Like passengers exiting a crowded subway car, the ammonia molecules must squeeze together to exit the compressor. As the ammonia molecules squeeze together, their weak bonds break and the ammonia boils. The compressor thus heats the ammonia, and begins to prepare the ammonia for its later duties.
The heat-exchanging pipes continue the task of preparing the ammonia. These pipes can be found near the top of the refrigerator, and are hot to the touch. This heat is the release of energy to the surroundings. As they make the treacherous journey through the heat-exchanging pipes, the freed ammonia molecules lose energy, similar to the subway patrons now climbing a flight of stairs. The ammonia molecules meet with friction- microscopic bumps, obstacles, and staircases- on the inside of the heat-exchanging pipes. The molecules meet these obstacles and release energy in the form of heat to overcome them. Ammonia loses heat to the kitchen as it traverses the heat-exchanging pipes. This is why the back of the refrigerator, and often the kitchen, is very warm.
The ammonia molecules themselves change as they exit the heat-exchanging pipes. As they release energy and slow down, they form bonds again. Our exhausted subway patrons are now able to regroup and meet up with their parties after exiting the train (compressor) and climbing the flight of stairs (heat-exchanging pipes.) The ammonia transforms from a heated liquid to a condensed liquid within the heat-exchanging pipes, a process called condensing [2]. The ammonia is now fully prepared for the second half of the refrigeration cycle.
The Refrigeration Cycle: Lights, Camera, Action
The expansion valve is the first instrument the prepared ammonia meets. Similar to the compressor (or exiting a crowded subway car), the ammonia molecules must again squeeze through a tiny hole. Again, the ammonia molecules get hot and boil, but this time they undergo a phase change to gas, a process called vaporization [2]. Immediately after the ammonia molecules exit the expansion valve, they are spread out among the coils of the refrigerator like subway passengers after exiting the train through a turnstile. Intermolecular bonds have been broken, but the ammonia is not a gas yet. It simply isn’t hot enough. The expansion valve essentially positions the ammonia molecules to make turning them into a gas very easy.
Here is where the food comes in. The separated ammonia molecules sense the heat from the room temperature ketchup bottles, day-old leftovers, and other contents of the refrigerator. The ammonia molecules draw this heat from the refrigerator contents and instantly boil (How). The food, robbed of its heat by the ammonia, is now cold. Gravity draws the ammonia from these crucial coils back through the compressor to begin its journey again [2].
Compression, heat release to the kitchen, expansion, and drawing in of heat from the food: these are the tasks ammonia performs repeatedly within a modern refrigerator to keep soda cold and food from spoiling.
What Is In Store For Refrigerators
This vapor-compression refrigeration cycle has kept milk fresh, lettuce crispy, and leftovers tasty for nearly a century. Nevertheless, enterprising companies are revamping the refrigerator. Many are capitalizing on technology that allows consumers to take control of the temperature of the refrigerator. Sub-Zero’s dual refrigeration system throws in an extra compressor for increased freshness and three evaporators (essentially expansion valves) to allow the user to control the temperature in different areas of the refrigerator [3]. Maytag also provides its customers with two thermostats to fine-tune temperature [4]. Whirlpool’s “In-Door Air Cooling System” directs air from the freezer into the refrigerator door to keep those items extra cold [5]. These Whirlpool refrigerators essentially have two refrigerants- air and ammonia.
Conclusion
The refrigerator maintains a silent but powerful presence in today’s home. His shiny skin and comforting hum masks his little secret: that he is an underappreciated marvel of engineering. New gadgets and processes may change him in the near future, but his contributions to food preservation and life itself will remain for all time.
References
[1] Krasner-Khait, Barabara. “The Impact of Refrigeration.” History Magazine. [Online]. Available: http://www.history-magazine.com/refrig.html, [Jul. 5, 2005[.
[2] D. Lenaker. “The Physics of Refrigeration.” University of Alaska, Fairbanks. Internet: http://ffden2.phys.uaf.edu/212_fall2003.web.dir/Dane_Lenaker/refrigerator2.shtm, [Jul. 5, 2005[.
[3] “Sub-Zero Reveals New 700 Series Models.” Sub-Zero. Internet: http://www.subzero.com/thelivingkitchen/press23.asp, [Jul 5, 2005].
[4] “Maytag ‘Freezer on the Bottom’ Refrigerators.” Maytag. Internet: http://159.41.169.144/pressroom/js/press.jsp?BV_UseBVCookie=Yes.
Whirlpool. 5 July 2005. http://www.whirlpool.com/catalog/product.jsp?src=Search&categoryId=96&productId=537, [Jul. 5, 2005].
[5] “Ammonia As A Refrigerant.” American Society of Heating, Refrigerating, and Air- Conditioning Engineers, Inc.. Jan. 17, 2002.
[6] S. Spielman. “Compressor Safety Criteria.” Internet: http://www.processcooling.com/CDA/ArticleInformation/Safe_Haven_Item/0,6582,106867,00.html, [Jul. 5, 2005[.
[7] R.S. Hartley. “Food”. West Virginia Association of Museums. Internet: http://www.museumsofwv.org/kids/pioneers_food.html, [Jul. 5, 2005].
[8] “How Does A Refrigerator Work?” California Energy Commission. Internet: http://www.energyquest.ca.gov/how_it_works/refrigerator.html, 2004 [Jul. 5, 2005].
[9] “Control Devices.” Sweethaven Publishing Services. Internet: http://64.78.42.182/sweethaven/MechTech/Refrigeration/coursemain.asp?lesNum=3&modNum=4, [Jul. 5, 2005].