Decaffeinated coffee is a popular beverage in cafes and homes throughout the world. There are several different methods used to decaffeinate coffee, including the direct method, indirect method, carbon dioxide method, and the Swiss Water Process. While the first three methods involve chemical treatment of the coffee beans, the last one—Swiss Water Process—does not involve any such treatment. Understanding the advantages and disadvantages of each method will help us to see why certain methods are more prevalent and advantageous in the decaffeination industry.
In order to avoid experiencing insomnia or hyperactivity, many people choose to drink decaffeinated rather than caffeinated coffee. However, seldom do coffee drinkers ask about the steps taken to produce that cup of decaf coffee. A common misconception is that decaffeinated coffee comes from naturally grown coffee beans (Fig. 1) that are already decaffeinated in nature. While that may become a reality in the near future, currently decaffeinated coffee is derived from artificial processes that involve great engineering depths.
Certain types of coffee beans naturally contain less caffeine than others, but to achieve the decaffeination standard of less than 1% caffeine, coffee beans must be treated chemically to remove the caffeine. Coffee beans are always treated prior to roasting, or when they are still green beans, and there are generally five ways to decaffeinate coffee beans: Roselius, direct method, indirect method, carbon dioxide method, and Swiss Water Process. Presently, only four of the methods mentioned above—Roselius, direct, indirect, carbon dioxide—are used to decaffeinate beans [1]. The Swiss Water Process, inheriting a proprietary name, is an innovative process that differs from the rest of the methods and comes with its own set of advantages and disadvantages. Despite the widespread usage of these methods, what may threaten decaffeination companies but cause excitement among insomniac coffee lovers is the discovery of naturally decaf beans—Decaffito [2].
Roselius
Roselius was the first decaffeination process developed. In 1903, Ludwig Roselius, a German coffee merchant, accidentally stumbled upon this method when his freight of coffee beans was soaked in sea water and lost much of its caffeine without losing much taste [3]. Thus, the Roselius process begins with a rinse-through of the unroasted coffee beans (green in color) in salt solution followed by repeated rinses (up to fifteen times) with benzene [3]. However, the use of benzene raised concerns for its health repercussions. The Roselius process is therefore no longer used.
Direct Method
The direct method, as its name implies, treats the beans directly with chemicals to remove the caffeine. Just like every other method of decaffeination, green coffee beans (unroasted beans) are soaked in water and then rinsed with either methylene chloride or ethyl acetate for ten or more hours to remove 99% of caffeine. Of the two types of chemicals, methylene chloride is more notorious for its carcinogenic properties, as confirmed by testing on lab rats, and even though the residual content of methylene chloride used is marginal enough to be acceptable by the FDA, in the long term it has the potential to cause harmful neurological effects, such as dizziness, loss of memory, changes in personality, and depression [4]. The other chemical in question, ethyl acetate, is often called the “natural process” because it is naturally produced in organisms such as ripened fruits in minuscule quantities [5]. However, despite ethyl acetate’s natural origins, the decaf process using ethyl acetate still involves larger quantities of the chemical than any naturally occurring process or organism.
Indirect Method
The indirect method is slightly more superior to the direct method in the sense that the chemicals do not come into direct contact with the coffee beans. First, the green or unroasted coffee beans are soaked in water to strip the flavor and caffeine from the beans. This water containing the flavors and the caffeine is then treated with the same chemicals used in the direct method to remove the caffeine. After the caffeine is removed from the water, the water is reinjected back into the beans to revive their flavors. The indirect method is less prone to criticisms of beans containing chemical residue, since the chemicals can be rinsed out before water reinjection. However, this poses an environmental challenge of the proper chemical disposal protocol: how should a decaf company ethically handle the decaffeinating chemicals afterwards? Eco-friendly disposal of the chemicals may impose additional costs to the firms, thus discouraging them from making environmentally conscious decisions [5].
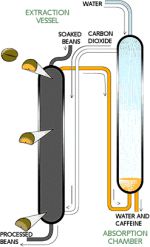
Carbon Dioxide Method
The carbon dioxide method involves soaking unroasted coffee beans in highly pressurized carbon dioxide that is compressed to two hundred times its normal atmospheric level [5]. The pressurized carbon dioxide removes the caffeine from the beans.
This method can be performed on a very large scale and is therefore the most widely used method for commercial-grade, less exotic coffee found in supermarkets. Mechanisms similar to the one shown in Fig. 2 can be found in a decaffeination plant implementing the carbon dioxide method.
The method can be summarized in several steps:
1. Green coffee beans are soaked in water.
2. Caffeine removal occurs in an extraction vessel suffused with CO2.
3. Decaffeinated beans at the bottom of the vessel are removed, dried, and roasted.
4. Recovery of dissolved caffeine occurs in an absorption chamber [6].
Unroasted beans go down from the small vessel at the top and as they enter the large vessel in the middle, carbon dioxide is compressed onto the beans, extracting the caffeine, leaving decaffeinated beans to fall through from the bottom vessel [6]. The downside of this method is that, unless it is used to achieve the appropriate large scale economies, the method requires exorbitant capital investments. The overhead electricity and labor costs are also steep [7].
Swiss Water Process
The Swiss water process may be a more desirable method since it is a chemical-free decaffeination process. A batch of unroasted coffee beans is first soaked in water to gently dissolve the flavor and caffeine out of the beans. This results in a batch of flavorless and caffeine-less beans, and water full in flavor and caffeine. The beans are tossed away, and the water is then passed through a filter to trap the caffeine, leaving water that is full in flavor and has no caffeine; this flavorful water has been termed the “Green Coffee Extract” by its inventors [8]. A second batch of green coffee beans is then soaked in the Green Coffee Extract (flavorful but caffeine-less water) to remove caffeine. However, this time around, since the Green Coffee Extract is saturated with flavors but not caffeine, the beans will not lose or gain any flavor and will lose caffeine to the Extract [5]. Now, the still flavorful water contains caffeine and is run through a filter again to remove the caffeine, making the water once again a Green Coffee Extract. The extract is used repeatedly for up to ten batches of beans. One cycle for one batch of beans, starting from creating the GCE to decaffeinating a batch of beans, takes approximately ten hours [8, 9].
The ingenuity of the Swiss Water Process is that, unlike its counterparts, it involves no chemicals in the decaffeination process. This method of decaffeination applies the concept of solubility and osmosis to decaffeinating coffee beans. Due to the absence of chemicals, attractive consumer terms such as “less artificial”, “more natural”, and “healthy” have become an asset of the Swiss Water Company that helps attract a large market segment of health conscious consumers. However, despite its healthy connotations, the Swiss Water Process can potentially be one of the more expensive processes since one batch of coffee bean goes to waste for every ten decaf batches. Another disadvantage to the Swiss Water Process is that flavors tend to be mixed up among batches since the Green Coffee Extract can carry a previous batch’s flavor [10]. From an industrial standpoint, the Swiss Water Process is not profitable enough to be widely used.
Decaffito—Naturally Decaffeinated Coffee Beans
Here is a disclaimer for avid decaf coffee drinkers: decaffeinated coffee is not completely void of caffeine. Decaffeinated beans still contain a caffeine content of 1% or lower (depending on different standards across countries) [11]. If decaffeinated coffee is not caffeine-free, a decaf coffee drinker is bound to experience the effects from caffeine eventually when larger quantities of the coffee are consumed. This opens up the floor to the question of whether 100% caffeine-free coffee would ever become a viable option. The answer to this question may very well lie in this one simple idea: coffee beans that are inherently caffeine-free. About five years ago, naturally decaffeinated coffee beans were found in a species of the Arabica bean family [12]. This new species of beans, known as
Coffea arabica, has been found to contain close to zero caffeine. In the future, it may be completely plausible to have
genetically modified caffeine-free plants that will yield zero caffeine content [13].
Conclusion
Many people enjoy the taste of coffee but prefer to avoid the physiological effects of caffeine. Thanks to basic feats of engineering, people throughout the world are able to enjoy decaffeinated coffee. Perhaps next time when you are at your local grocery store picking up some freshly ground decaf beans, you will pay closer attention to the engineering process involved in producing your decaffeinated coffee.