In the Fall 2003 semester, Marianne Case was a junior majoring in Chemical (Biochemical) Engineering with a minor in Neuroscience. She is from Tacoma, WA. She is active in the USC branch of AIChE and enjoys playing the violin.
The skin is a complex organ with many layers. Its structure is designed to minimize moisture loss from the body while preventing foreign materials from entering. To accomplish these functions, the skin must have a protective covering of lipids, or oil-soluble molecules. Exposure to everyday conditions can strip the skin of its protective lipid covering. Therefore moisturizers containing some oil-soluble components are often used to restore the skin to its natural condition. Most moisturizers use a mixture of oil and water soluble components called an emulsion. More recently, researchers have begun incorporating liposomes, or small spheres of lipids, into moisturizers. Not only are these bubble-like structures useful for restoring the skin’s protective lipid layer, but they can also carry active ingredients inside of them, increasing the potency of some cosmetic products.
Introduction
Nothing hinders a smile like dry skin. Skin must maintain a certain amount of water inside to remain stretchy and feel comfortable. However, splashing water directly onto your face makes it feel drier than before. So how do moisturizers add water to your skin and keep it there? They require a combination of water and oil to work properly. A few different techniques for delivering moisturizers to your skin exist. Moisturizers often incorporate emulsion systems, which are mixtures of oil and water. They may also add a more recently developed technology that uses vesicles, or microscopic “bubbles” made out of biological components. In this article we will first describe emulsions, and then go into detail about a kind of vesicle called a liposome, which is commonly used in moisturizers. We will also explain why utilizing a vesicular system is generally superior to using emulsions only. First, however, a brief review of the skin’s components is helpful.
The Skin
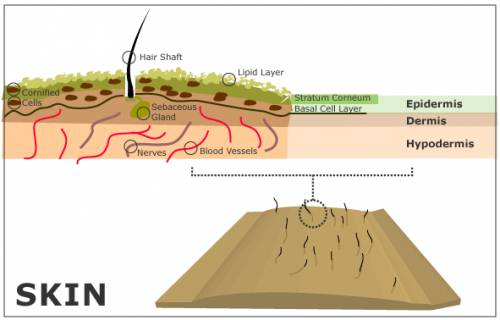
The skin is made up of three main layers (see Fig. 1): the epidermis, the dermis, and the hypodermis [1].
As the deepest layer of skin, the hypodermis contains a layer of fat. It also houses blood vessels and nerves that are generally larger than those found in the dermis layer [2].
Within the dermis lie the blood vessels, nerves, and hair shafts, as well as sweat and sebaceous glands. The sebaceous glands produce an oily substance called sebum and secrete it into the hair shaft. Sebum migrates up to the top of the skin through the hair shaft, where it covers the outer layer of the epidermis, providing a protective barrier for the skin from the outside world. It consists of fatty acids, cholesterol, waxes, triglycerides, and a variety of other substances [1]. A small coating of sebum will protect the skin effectively. An excess of sebum leads to overly oily skin and can cause acne.
Within the epidermis are multiple sublayers. On the very surface of the epidermis lies a layer of fat [1]. Just beneath the fat is a layer of cornified cells, called the stratum corneum [1]. Beneath the cornified cells lies the horny layer, which consists of three sublayers [1]. At the bottom of the epidermis lies the basal layer, where cells are created. These cells then migrate up through the layers of the skin all the way to the stratum corneum, where they fall off the body [1]. This upward cell movement helps defend the skin from harmful invaders by acting like an ocean current, moving foreign debris up and out of the skin.
Why the Skin Needs Moisturizers
The epidermis is most relevant to this discussion, since cosmetic products target the cells in that section and are generally not able to migrate past it into the dermis [1]. Most cosmetic products that claim to improve the skin’s appearance and texture act on the stratum corneum layer of the epidermis [3] because it is the outermost layer of the skin — when it is scaly, the skin appears rougher. These products often fill in gaps between the cornified cells of the stratum corneum, creating the appearance of smoother skin. They may also serve as occlusives, meaning that they create a barrier on the skin through which water can not permeate. It may seem disadvantageous to apply such a barrier to the skin because it would prevent the skin from absorbing water in the air. The water content inside the skin, however, is generally much higher than that of the air. The moisture content in the stratum corneum should be about 15% [2]. The water content increases deeper in the skin, reaching about 80% in the dermis [4]. Therefore, in the absence of a barrier, water is much more likely to leave the skin than to enter it. Applying a barrier helps the skin retain more water.
In a perfect world, skin would be able to keep itself hydrated. In reality, however, irritants such as harsh cleansing products and excess exposure to environmental elements can upset the skin’s natural balance by removing its lipids. When these protective oil-based products in the skin are removed, the effectiveness of its barrier decreases. Water can evaporate from the skin more easily. This problem is further compounded by the fact that the resulting water loss will decrease the barrier’s ability even more.
Water is necessary for the skin to maintain its flexibility; when skin is overly dry, it loses its ability to stretch, causing it to crack and peel more easily. Faster peeling means skin cells are being shed more rapidly, which triggers an increase in the rate of cell production in the basal layer. When the process of cell growth, migration, and shedding is accelerated, the barrier is significantly weakened because the cells providing the barrier have not had time to fully mature [5]. Generally, as the cells rise to the surface of the skin, they lose all their components except their protein skeleton structure [6]. It is this layer of protein which provides the barrier against water loss. When the process of cell turnover is sped up, the cells reach the top of the skin before they have completely flattened and lost the rest of their components. These immature cells cannot prevent water from moving across them as well as the older ones can. Therefore, water is lost from the skin at a faster rate, and the skin is less able to defend against foreign invaders [5].
However, water alone will not increase the skin’s moisture level. It can easily evaporate off the surface of the skin [3]. A protective lipid coating is necessary to prevent water from evaporating off the skin. A good moisturizer must be able to supplement this barrier with the incorporation of hydrophobic components. Ideally, a product will not provide a completely occlusive barrier for the skin, but will offer partial protection. By partially protecting the skin from the air, a product will not interfere with the epidermis’ ability to manufacture its own lipids to restore the stratum corneum’s natural barrier function [7]. This way an improved barrier function will still be observed after the product is removed [3]. Therefore, it is important that moisturizers have an oil component. However, oil-based formulas are messy and awkward to use, so moisturizers are generally water-based with some hydrophobic components incorporated.
Emulsions
An emulsion is a “mixture” of oil and water (see Fig. 2). Because of their chemical composure, oil and water do not naturally mix. The atoms within water have slight negative and positive charges on them, and so they are drawn to other charged particles. Water molecules are not able to bind to the uncharged molecules in oil, so they cannot properly mix with the oil and instead form a separate phase when placed in the same container. Therefore, a chemical that attracts both oil and water must be added to the mix so that the oil and water phases will combine. This chemical is known as a surfactant. A surfactant must have two parts, one that binds water and one that binds oil. Surfactants are found in many household products. One of the most common examples of a surfactant is dishwashing soap, which contains chemicals that bind both water and oil. When combined with water, the mixture is able to bind the greasy residues on dishes, pulling them off as the mixture runs over the dish. Water alone would simply slide over the oil, not able to remove it from the dish.
How Emulsions are Formed
Water, an oil-based component, and a surfactant must undergo a process that thoroughly mixes them together. To ensure that the final product is water-based, the amount of water added to the mixture should be somewhat greater than the amount of oil-soluble substance added, so that mixing will produce an aqueous phase with small amounts of oil dispersed throughout. If too much of the oil-based product is added, the water and surfactant phase will not be able to “dissolve”? it all, and a separate oil phase will form. Alternatively, the emulsion will instead become oil-based, with small amounts of water mixed into it. The choice of surfactant affects the ratio of water to oil that can be mixed and still remain in one phase. Some surfactants are able to bond with more oil molecules than others, meaning that more oil can be dissolved in a given amount of water.
How Emulsions Benefit the Skin
The skin needs oil-based products, but they are messy to apply. Imagine having to smear petroleum jelly on your skin to moisturize it. Emulsions provide a convenient way to deliver oil-based products without the mess of handling them. After the product is applied, excess water will evaporate from the skin, leaving a layer of the oil-soluble product behind with some water [3]. In addition to delivering the oil-based product, the surfactant may bind the remaining water to the skin to increase its water content. The surfactant is able to do this because it is also still bound to the lipid or oil-based product that forms the protective barrier for the skin.
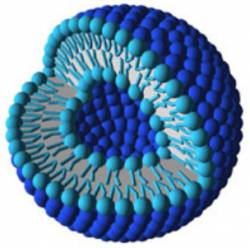
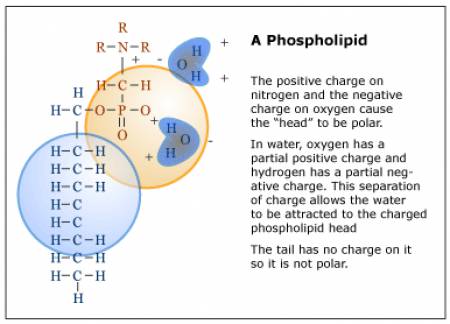
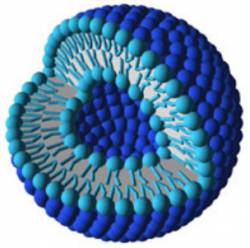
Liposomes are small vesicles, ranging in size from 15 nanometers to 3.5 micrometers (see Fig. 3) [8]. The vesicle walls are made up of one or more concentric phospholipid bilayers. A phospholipid is a biological molecule with a hydrophilic head and a hydrophobic hydrocarbon tail. The head is somewhat polar and so it attracts water, which is also a polar molecule (see Fig 4). The head of a phospholipid and a water molecule are like oppositely-charged magnets that attract each other. The phospholipid’s tail, however, is not charged, and so it does not exert any attractive force towards the water. In a bilayer, there are two rows of phospholipids. Their tails point inward to the middle of the layer and their heads point outward, so that the surface of the bilayer on the outside and inside of the vesicle is hydrophilic. These vesicles may be empty or filled with an “active agent”? such as a vitamin or moisturizing compound that is meant to be released when the vesicle penetrates the skin. The agent may be either water-or oil-soluble.
How Liposomes are Formed
Liposomes can be created using a variety of techniques. The method that one selects depends on how many bilayers are wanted in the vesicular membrane and on the fragility of the active agent to be enclosed. A more fragile active agent would need to be surrounded by a greater number of layers. One common technique for creating multilamellar liposomes is explained below.
The vesicle wall is made up of lipids, or fat soluble molecules (Fig. 4). First, the lipids are dissolved in an organic solvent [10]. Any number of solvents will work; one example is t-butanol [9]. This solution is left alone until all the organic solvent has evaporated, leaving a thin coating of the dried lipid on the walls of the flask [10]. Most organic compounds boil at a much lower temperature than water, and so they may evaporate readily when left out. Then a water soluble active agent solution is poured into the flask [10]. Active agents can be nutrients, vitamins, or other additives used to improve the skin’s texture and appearance. The flask is swirled to loosen the lipids from the flask wall and break up large clumps of lipids. The lipid pieces will automatically form closed vesicles, trapping some of the active agent inside [10]. The flask is then left to equilibrate for a few hours. Afterward, any active agent that was not trapped by the lipid vesicle formation must be washed away [10]. The liposomes will then be immersed in the moisturizer, which contains little or no active agent [10]. This procedure works fine for making small amounts of product, but when the process is “scaled up” by a company to mass produce a moisturizer, it is not feasible to use the evaporation technique to coat a container with dried lipid. Instead, a freeze drying method is used to coat flask walls with lipids [9].
How Liposomes Benefit the Skin
To understand the effect liposomes have on the skin, let’s first examine what an empty liposome does when it comes into contact with the skin. The liposome migrates into the cornified layer, breaking as it comes into contact with the horn-like cells, much like a balloon that floats into sharp shrubbery and then pops. These pieces of liposome lipids will migrate into the stratum corneum [8], forming an occlusive barrier that slows water loss through the skin. The hydrophobic portions of the lipid will “repel” water. Since it cannot easily cross over the hydrophobic lipid tails, the water will remain within the layers of the skin. Here, the liposome walls are serving the same function that oil-soluble products serve in emulsions.
Now, imagine this liposome has an “active agent”? housed inside its vesicular membrane. The agent will escape from the vesicle when it breaks in the cornified layer. After the active agent is released, it will migrate further into the skin. The active agent will be more concentrated inside the skin than it would if it were evenly dispersed throughout the entire product for two reasons. First, a concentrated amount of the agent is introduced directly inside the skin when the vesicle breaks. Second, the vesicular membrane has again formed an occlusive layer at the top of the stratum corneum, which seals the active agent into the skin [8].
Benefits of Using Vesicular Systems
The use of vesicles to deliver active agents to the skin has a variety of advantages over distributing active agents throughout the entire product. The increased retention of the active agent within the skin as described above is one of them. Storing the active agent inside a vesicle also protects it within the product until it is ready to be used by keeping it in a more stable environment. It is not subjected to a high concentration of the aqueous medium when inside liposomes, and therefore it is less susceptible to degradation [11]. Also, because the vesicles travel slightly into the skin before releasing the active agent, the layers of skin above the release point are not exposed to the agent. Since most agents are not targeting these upper layers anyway, the use of vesicles makes them more effective and less likely to have side effects [11].
Another advantage liposomes hold over emulsions is that they do not contain as many surfactants. Once surfactants come into contact with the skin, they often emulsify the sebum on the top layer. This is not always desirable, as the sebum could then be stripped away, leaving the skin without its protective barrier and therefore prone to irritation [3].
Products that can benefit from using liposomes include moisturizers that contain components such as Vitamin A (Fig. 5). Occasionally, derivatives of Vitamin A may cause irritation when applied to the skin [5]. However, Vitamin A is useful in repairing the barrier when the skin’s cell turnover rate has accelerated due to moisture loss. It can help the cells mature and grow larger, which improves the skin’s moisture content and its ability to fight off foreign particles [5]. Therefore, it may be useful to incorporate Vitamin A into liposomes, which can migrate slightly into the skin and then release their contents. That way, the Vitamin A is carried to where it can actually be of use. Areas that do not need Vitamin A (closer to the surface of the skin) are subjected to lower concentrations of it, causing less irritation.
Liposomes have also improved the duration of tans created by self-tanners. When the chemicals that dye the skin are incorporated into the vesicles, a lower concentration of the dye is required to produce the same color intensity [8]. Also, the tan produced from a liposome-containing product is more resistant to washing of the skin than one produced from a simple emulsion product [8].
Conclusion
Emulsions and vesicular systems both serve the important function of retaining or increasing water content in the skin by incorporating some hydrophobic components, and the two processes are not mutually exclusive. While emulsions of oil and water have been used for centuries, liposomes have appeared only in the last few decades. Even when liposomes are used in a product, the product will most likely contain an emulsion, too. Liposomes, however, have been shown to have advantages that emulsions do not, making them a wise addition to many products.
By concentrating special agents in the skin layers, which actually benefit from them, liposomes can both increase their effectiveness and decrease the risk of irritation. They also require a smaller amount of these active agents than emulsions, which must have the agents dispersed evenly throughout the whole product. Even when liposomes contain water soluble active agents, they still provide a partially occlusive barrier to the skin. This barrier function is accomplished by the phospholipids that make up the vesicle walls of the liposome. The occlusive barrier will prevent both water and the active agent from leaving the skin, another way in which active agent effectiveness is increased.
Emulsions will probably always serve a vital function in cosmetics and in a wide array of other products, ranging from foods to pharmaceuticals to household products. Liposomes are an alternative to emulsions — when their use is appropriate in a cosmetic product, they often increase the product’s benefits. As a more recent development than emulsions, their full value to the cosmetics industry has not yet been realized. The use of liposomes in cosmetics can be expected to increase in the future.
References
-
- [1] W. A. Poucher. Perfumes, Cosmetics & Soaps, vol. 3. 8th ed. Rev. G.M. Howard. New York: Chapman and Hall, 1974, pp. 311-313.
- [2] J. Gray. (1997). “The World of Skin Care.” [On-line]. pp. 10-39. Available: http://www.pg.com/science/skincare/Skin_tws_toc.htm [Oct. 15, 2003].
- [3] Z. K. Draelos. Atlas of Cosmetic Dermatology. Philadelphia: Churchill Livingstone, 2000, pp. 3-86.
- [4] “Skin Education.” Internet: http://www.h2oplus.com/retail2002/SkinEducation.aspx, 2003 [Dec. 1, 2003].
- [5] D. H. Maes and K. D. Marenus. “Main finished products: moisturizing and cleansing creams.” Cosmetic Dermatology. Ed. Robert Baran and Howard I Maibach. Baltimore: Martin Dunitz Ltd., 1994. 77-88.
- [6] M. Courtenay. (1998, Feb.). “Preparations for Skin Conditions.” Nursing Times. [On-line]. Vol. 94(7), pp. 54-55. Available: http://www.ncbi.nlm.nih.gov/pubmed/9536736 [Dec. 1, 2003].
- [7] P. M. Elias and M. L. K. Williams. “Structure and Function of the Stratum Corneum.” Internet: http://www.aad.org/education/stratumcorneum.htm, 2003 [Dec. 1, 2003].
- [8] M. Seiller et al. “Vesicular systems and multiple emulsions in cosmetology.” Cosmetic Dermatology. Ed. Robert Baran and Howard I Maibach. Baltimore: Martin Dunitz Ltd., 1994, pp. 27-36.
- [9] L. S. Rao. “Preparation of Liposomes on the Industrial Scale: Problems and Perspectives.” Liposome Technology,vol. 1. Ed. Gregory Gregoriadis, 1st ed, Boca Raton, FL: CRC Press, 1984, pp. 247-259.
- [10] D. Chapman. “Physicochemical Properties of Phospholipids and Lipid-Water Systems.” Liposome Technology,vol. 1. Ed. Gregory Gregoriadis. 1st ed. Boca Raton, FL: CRC Press, 1984, pp. 1-18.
- [11] “Nanotechnology Research Laboratory Information.” Internet: http://www.nanotech21.org/eng.htm [Oct. 15, 2003].
- [12] J. de Gier. “Osmotic Properties of Liposomes.” Water Transport in Biological Membranes. Vol. 1. Ed. Gheorghe Benga. Boca Raton, FL: CRC Press, 1989, pp. 77-98.
- [13] C. J. Kirby and G. Gregory. “A Simple Procedure for Preparing Liposomes Capable of High Encapsulation Efficiency under Mild Conditions.” Liposome Technology, vol. 1. Ed. Gregory Gregoriadis. 1st ed. Boca Raton, FL: CRC Press, 1984. 19-28.
- [14] Skin Care Forum 27. “Schematic Diagram of the Skin.” Internet: http://www.scf-online.com/english/27_e/27_e_pr/frontpage27_e_pr.htm, Oct. 2001 [Oct. 15, 2003].
- [15] G. Solomons and C. Fryhle. Organic Chemistry. 7th ed. New York: John Wiley & Sons, Inc., 2000.
- [16] T.F. Tadros. “Some Applications of Surfactants in Biological Systems.” Surfactants. Ed. Th. F. Tadros. Orlando, FL: Academic Press, 1984, pp. 323-336.
- [17] “Technologies: Liposomal Drug Delivery Systems.” Internet: http://www.northernlipids.com/index2.htm, 2002 [Oct. 15, 2003].
- [18] B. Vincent. “Emulsions and Foams.” Surfactants. Ed. Th. F. Tadros. Orlando, FL: Academic Press, 1984, pp. 175- 196.