The field of tissue engineering has seen significant improvements in the past 10 years, much of which is due to the development of tissue scaffolds. These 3-dimensional, porous structures are perfectly suited for cellular attachment and growth due to their physical similarities to the native extracellular matrix. The ability of scaffolds to be strong yet flexible further increases their application versatility. When constructing scaffolds for implementation, two elements are necessary: a cell-growth base derived from a tissue culture and the biodegradable, polymer-based scaffold itself. When these two elements are combined and inserted into the patient, tissue repair occurs through cellular proliferation. Such scaffolds are currently used in the regeneration of cartilage, bone, and elements of the cardiovascular system, with some electrically-conductive scaffolds even tailored for neural repairs. While these polymeric scaffolds have proven applicable in the above-mentioned fields, recent research suggests improvements on the current technology. Rather than a composition of polymer blends, new “biological scaffolds” use collagen fibers derived from either animal or human donors that can assemble spontaneously into scaffold structures. These biological scaffolds have shown to decrease immune system rejection and increase regenerative success, making them the next great technological development in tissue engineering.
Introduction
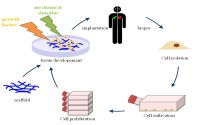
In the mid 1980s, tissue engineering was established as the next major biological breakthrough. As relevant technology developed, it became plausible for engineered tissues to replace organs and other living cells that had been damaged or lost. Successful regeneration showed exceptional promise with the use of biocompatible materials that function as connectors across an injured area [1]. These “biological bridges” allow for cell proliferation and, thus, reattachment and organ growth. With this goal in mind, increased funds and research have been invested in developing instruments for cell growth with the most effective and useful medium being the scaffold (Fig 1).
As would be expected with a name referencing construction, most tissue scaffolds physically resemble building scaffolding. Tissue scaffolds appear as 3-dimensional cubes supported by cross-linking. The resulting porous structure allows cells to grow and attach to regular tissue, as pictured below.
Many different materials have been used in the development of this technology, including metals, calcium phosphate ceramics, glass, and silk proteins. While ceramic and glass scaffolds are still used in some bone and cartilage engineering, currently the most widely-used scaffold materials are polymers, or chains of molecules that interact chemically to form a substance. Certain polymer compositions are more suited for particular fields, but most polymers used have biodegradable abilities. Biodegradability is important for the administration of scaffolds in the body because the removal of an implanted device is considered to be one of the most dangerous surgical procedures[2]. With biodegradable scaffolds, the scaffold can be absorbed by the body, eliminating the necessity for removal procedures. While certain tissues (such as bone) may require permanent support, the majority of soft tissue transplants are actually negatively affected by long-term foreign bodies. Thus, the development of polymer scaffolds has made these implants even more versatile.
The most significant parts of the tissue scaffold are the pores. Because scaffolds are cell growth instruments, it is important to maximize the surface-area to volume ratio of the structure. However, the engineering task of minimizing the structure of the instrument while maximizing available space for cells to grow is challenging. This issue is often addressed through strict maintenance of pore size. While the number and orientation of the pores may vary, most scaffold pores fall between 50 and 500 micrometers in diameter [3].
The size within this range is determined by the type of tissue repair. With hard tissue engineering, which is generally confined to the regeneration of bone, scaffold pores are often large and highly interconnected, allowing cells to efficiently grow all throughout the scaffold while maintaining a rigid structure. [4] Scaffolds for soft tissues, such as those for nerve and cardiac fibers, also have highly interconnected pores, but these pores are often smaller. Scaffold with smaller pores favor flexibility and a greater surface area rather than rigidity, as extensive structural support is unnecessary for soft tissue. [4]
The characteristic properties of scaffolds, strong, flexible, and porous, are significant to their role as biological substitutes. This is because the scaffold must mimic the extracellular matrix (ECM) of the body. The ECM is a network of connective tissue that supports and anchors body cells, similar to the role of scaffolds. Like the ECM, scaffolds serve primarily as structural support, providing the shape and rigidity of the physical tissue. Additionally, both scaffolds and the ECM are important in promoting cell proliferation and differentiation, allowing tissue to regenerate and form additional blood vessel connections [5]. Because tissue scaffolds are constructed like existing ECMs and use biocompatible materials, they are easily integrated into the body. These characteristics suggest that scaffolds will serve important roles in the current and future practices of tissue engineering.
Tissue Scaffolds in the Making: Production and Insertion of Scaffolds
Two main components are required to complete a tissue donation using scaffolds: a cell growth base and the biocompatible scaffold itself.
Cell Growth Base
While the type of cells cultivated depends on the specific donor tissue, a few basic elements are consistent when creating all cell growth bases. The primary goal in preparing a cell culture is actually to obtain a single layer of tissue that can serve as baseline for cell growth. Generally, the desired tissue is obtained from a cadaver, but technology is progressing to allow tissue recipients to self-donate to eliminate the chance of rejection. Following the retrieval, the useful cells are first isolated and then cultured in incubators for about 6 days [1]. The piece of tissue is then thoroughly cleaned with alkaline substances and deionized water to remove all blood and fat that may have developed during the incubation period [1]. What remains is the collagen base and inner-most tissue layer. (Vacanti) This simple cell layer is a schematic of the scaffold construction process. Cells of the cultured tissue can then grow on top of the base layers to create a small culture for implantation.
Scaffolds Creation
Creating the scaffold is the next step. Tissue engineers have developed efficient manners of creating these 3-dimensional shapes. While some techniques involve polymers that can naturally assembly into porous structures, these processes are less effective than those that use Teflon molds [1]. In this process, the mold is filled with biodegradable polymer powder and microscopic spheres of gelatin [6]. Pressure is applied to the mixture and temperature is set to about 35 degres Celsius, causing the polymer particles to bond with one another. Following this process, the mold is removed and the mixture is placed in water, causing the gelatin to leak out and leave behind pores where the microscopic spheres once were. This porous scaffold is then immersed in a solution of proteins to make the scaffold more biocompatible and active as a cell growth medium [1]. When the scaffold is dried it is considered complete, as it has become porous and assumed the same shape as the mold.
Through this process, the scaffold attains all of the necessary characteristics: porosity to favor tissue integration; biodegradability so that tissue can eventually replace the scaffold; and biocompatibility to reduce adverse responses to the implant [6]. These characteristics combine to make a scaffold the perfect instrument for cell attachment and proliferation.
Following the creation of the scaffold, the cultured cells are given time (about 4 to 5 days) to develop both an ECM and a thin layer of viable cells throughout the pores of the scaffold in a process called cell seeding. The environmental conditions for the cells to adhere to the scaffold must be both wet and warm, so a humidified incubator is used to foster growth on the medium [1]. Following this step, the prepared scaffold is implanted into the subject. Due to the scaffold’s biodegradable and conductive nature, it can both improve and direct tissue growth while it is still present in the body. This allows the creation of new body tissue using the scaffold as a support [1].
Current Applications of Scaffolds
Through this process, the opportunities for cell growth are limitless. Such scaffold mediums can be used to cultivate and proliferate many cell types. Research on the use of scaffolds in the fields of muscle, blood vessel, and bone cell replacement have shown progress, while extensive studies in tissue engineering suggest a promising future for scaffolds in the fields of cartilage, cardiac, and neural repairs.
Cartilage
Repairing cartilage has long been a challenge for physicians. Due to its virtual lack of blood and nerve supply, cartilage cells are unable to migrate to the site of injury and thus cannot repair themselves naturally. This has led to surgical injury repair involving metal implants, making these surgeries dangerous to the patients due to the introduction of large foreign objects, risking patient-immune rejection. The use of scaffolds in this field, however, largely eliminates these risks and has even proven more effective.
Polymers of dense fibers are used in cartilage scaffolds because these polymers are most conducive to the cartilage matrix [7]. These scaffolds maintain the structure and appearance of natural cartilage cells while also limiting the movement of inflammatory proteins to the implantation site, reducing unwanted immune responses [2]. Furthermore, unlike the majority of other materials used in cartilage repairs, scaffolds can be tailored specifically for the site of injury. For example, a scaffold designated for cartilage damage of the knee can have spherical or elliptical pores and increased rigidity to increase cartilage growth in that area, whereas scaffolds for cartilage damage in the shoulder would be more elastic [1]. This versatility has made cartilage scaffolds usable in almost every cartilage repair. However, the future of scaffold use in this field and others appears even more promising.
Cardiology
Heart muscle and blood vessels have drastically different anatomies than cartilage, and therefore require another approach to creating scaffolds. The classic porous structure of cartilage scaffolds are not effective for cardiology, as the dynamics of the ECM in the heart are distinct. When creating these scaffolds, a blend of gelatin and biologically-based polymers is used in a mold that mimics the heart muscle structure, making these scaffolds appropriate for the growth and proliferation of heart muscle cells [5]. Blood vessels require a slightly different scaffold approach than heart muscles; blood vessel scaffolds usually use two different polymers in their blend and must be placed in cylindrical molds based on the width of the blood vessel it is replacing [1]. These characteristics make cardiology scaffolds perfectly tailored for their specific uses.
Neurology
While cartilage and cardiac care have shown immense improvements, neural repair is one of the most revolutionary applications of this technology. Most neural surgeries are unable to maintain the electrical and physiological interactions of natural nerve units, but scaffolds can be tailored to have these characteristics. Scaffold specified for neural units are composed of hydrophilic (water-attractive) polymer blends because of their affinity for charged particles. The hydrophilic components are then charged through an injection when soaking the scaffold in biocompatible materials. This process makes the scaffold able to relay electrical signals to adjacent neurons when in the body, something that was previously unthinkable in neural repairs [1]. With neural units, native tissue is the best environment for regeneration, and as a result nerve grafts from the recipient patient are used in the scaffolds [7]. This process increases the probability of successful nerve repair while reducing the chance of patient rejection.
Biological Scaffolds as the Future of this Technology
Extensive research has been dedicated to furthering this scaffold technology. Much of the focus has been placed on reducing, and possibly eliminating, the chance of recipient rejection. With this aim, biomedical engineers have discovered a promising new construction: scaffolds solely composed of biological components. These so called “biological scaffolds” are the newest addition to tissue engineering, with research on their development beginning only three years ago. Rather than a composition of polymer blends, these scaffolds use collagen fibers derived from either animal or human donors, with the donation occasionally being from the recipient [8]. Currently, progress is being made on research in the United Kingdom using such biological scaffolds as vascular patches, knee cartilage, and tendons. Researchers have found that this technique has increased transplant longevity and dramatically increased the acceptability rate because the scaffolds are biological in nature and thus accepted by the recipient’s immune system. Because the scaffolds are not rejected, the patient’s cells can populate the new biological scaffolds, allowing the transplanted sections to be repaired like normal tissue [9]
The primary reason that these biological scaffolds appear to have greater promise than those currently in use is that they not only maintain, but improve upon, their similarities to the ECM. Because of their biological origins, these scaffolds do not require molds. The collagen components naturally bind together to form an ECM identical to their anatomical equivalents [9]. This fact is particularly true in the field of neurology, in which biological scaffolds have already begun to prove themselves. These neural scaffolds are often composed of self-assembling proteins. As the primary components of the neural environment, these proteins naturally form a structure conducive to neural growth when in an aqueous environment [10].
Conclusion
While scaffolds have been used for the past 25 years, great strides have been made in there development over the last ten years. With footholds in cartilage and bone repair, neurology, and cardiology, these cell-growth mediums are likely to become functional in almost every medical practice. With the further development of biological scaffolds, the possibility of safer and improved tissue regeneration is on the horizon, all courtesy of biology’s tiny construction project.
References
-
- [1] X. Li et al. “Recent Patents on Polymeric Scaffolds for Tissue Engineering.” Recent Patents on Biomedical Engineering 2, pp. 65-72, 2009.
- [2] J. Iwasa et al. “Clinical application of scaffolds for cartilage tissue engineering.” Knee Surgery, Sports Traumatology, Arthroscropy, vol. 17.6, pp. 561-777 561-577, 2009.
- [3] R. Dittrich et al. “Mineralized Scaffolds for Hard Tissue Engineering by Ionotropic Gelation of Alginate.” Advances in Science and Technology, vol. 49, pp. 159-164, 2006.
- [4] J. M. Karp et al. “Scaffolds for Tissue Engineering.” Materials Research Society Bulletin , vol. 28 pp. 301-306, 2003.
- [5] B.P. Chan and K.W. Leong. (2008). “Scaffolding in tissue engineering: general approaches and tissue-specific considerations.” European Spine Journal. [Online]. Vol. 17.4, pp. 467-479. Available: http://www.ncbi.nlm.nih.gov/pmc/articles/PMC2587658 [Nov. 13, 2008].
- [6] E. Sachlos and J.T. Czernuszka. “Making Tissue Engineering Scaffolds Work. Review of the Application of Solid Freeform Fabrication Technology to the Production of Tissue Engineering Scaffolds.” European Cells and Materials, vol. 5, pp. 29-40, 2003.
- [7] R. Ackbar et al. “Scaffolds for Skeletal Tissue Engineering.” The University of Sheffield.. [Online]. Available: http://iadr.confex.com/iadr/bsdr07/techprogram/abstract_95076.htm 2007.
- [8] M. P. Vacanti and C. A. Vacanti. “Biological Scaffolding Material.” U.S. Patent 7,319,035, Jan. 15, 2008.
- [9] (2010). http://www.sciencedaily.com/releases/2010/07/100713191219.htm“New Generation of Biological Scaffolds.”Science Daily. [Online]. Available: http://www.sciencedaily.com/releases/2010/07/100713191219.htm [June 18, 2011].
- [10] F. Blanchard. “Biological Scaffold Allows Nerve Cells to Grow and Form Connections.” The Whitaker Foundation News.Internet: http://web.mit.edu/lms/www/HTML%20papers/000630whitaker.htm June 30, 2000.