Andrew Wong was a junior studying Biology and Computer Science at the University of Southern California in the fall of 2003. Aside from his pre-medical pursuits, he enjoys relaxing at the beach, singing a cappella and snowboarding.
Two retinal diseases, Age- Related Macular Degeneration (AMD) and Retinitis Pigmentosa (RP), are the leading causes of blindness in individuals over the age of 65. Despite various treatments such as gene therapy and retinal tissue transplant, physicians have thus far been unable to combat the blinding effects of these diseases. With the knowledge that AMD and RP affect only photoreceptor cells, however, a new approach centering on the retinal neurons’ ability to be electrically stimulated is being developed. In this method, physicians propose the implantation of a retinal prosthesis device designed to replace photoreceptor cells that have been damaged by retinal disease. The innovations in silicon microchips and microcircuitry have given ophthalmologists the means to create such prosthetic devices. Current trials have been successful, restoring the patients’ abilities to distinguish contrast and differing light patterns. In current trials, patients have been able to distinguish contrast and differing light patterns. These retinal prostheses show remarkable promise for future clinical applications completely restoring vision in patients suffering from AMD and RP. This article discusses two new prosthetic devices: the Artificial Silicon Retina (ASR) and the Multiple-Unit Artificial Retina Chipset (MARC). This article focuses primarily on the development, design, and implementation of an experimental device called the Artificial Silicon Retina.
Introduction
Over 30 million people in the world today are affected by retinal diseases. In fact, Related Macular Degeneration (AMD) and Retinitis Pigmentosa (RP) such as Age-Related Macular Degeneration and Retinitis Pigmentosa are the leading causes of blindness in individuals over the age of 65. While physicians have attempted to treat these diseases using various drugs and therapies, such approaches have as yet to succeed.
In spite of this, recent developments in microtechnology and biomedical engineering, however, could potentially restore sight to millions of individuals suffering from retinal disease. Biotechnology is a new approach in this particular medical field; Such advancements in microcircuitry and biocompatible implant materials have given physicians and scientists the opportunity to develop prosthetic devices that are capable of mimicking the function of damaged retinal cells. In fact, two recently developed prostheses, the Artificial Silicon Retina (ASR) and the Multiple-Unit Artificial Retina Chipset (MARC), show immense potential for restoring vision to AMD and RP patients where other treatments have been unsuccessful.The development and clinical application of one such device called the Artificial Silicon Retina will be the focus of this article.
Background
The Human Eye
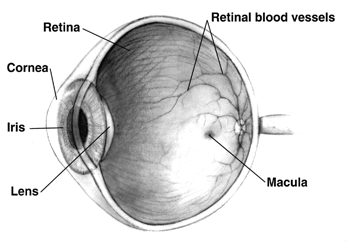
The human eye consists of many different structures that work collectively to “preprocess” and “send” images to the brain (see Fig. 1). Light first passes into the eye through the cornea and lens of the eye, which focus the light upon onto the posterior portion of the eye called the retina.
Distributed throughout the retina are two types of photoreceptor cells: rods and cones [1]. These cells convert the initial light energy that enters the eye into electrochemical impulses signals and initialize the propagation of visual information to the brain via the optic nerve. More specifically, the rod cells respond to varying degrees of light and dark, while cone cells respond to color stimuli. The area most densely populated with photoreceptor cells is called the macula. This region is responsible for receiving and processing the most detailed portion of our visual field and is also the region that is most affected by retinal diseases [2].
Retinal Disease
The most common retinal diseases are Age-Related Macular Degeneration (AMD) and Retinitis Pigmentosa (RP). These progressive diseases are progressive and cause the irreversible loss of photoreceptor cells. In the primary stages of AMD, nutrient and waste transport is slowed in the tissue beneath the retina. With insufficient nutrient supply and continual build-up of waste, the overlying rods and cones become damaged. In the more severe stages of AMD, new blood vessels will grow underneath the retina in attemptss to provide nutrient and waste transport; however, these new vessels tend to leak blood and waste fluids into the retina, completely damaging the surrounding tissue [3].
RP, a hereditary disease with effects similar to AMD, differs in that it affects mainly the rod photoreceptor cells (see Fig. 2). As the rod cells gradually deteriorate, RP patients lose sensitivity to contrast and experience difficulty seeing at night. Progression of the disease can lead to complete blindness [4]. While the clinical courses of AMD and RP vary, both result in the loss of photoreceptor cells, causing severe light insensitivity and, ultimately, blindness. Treatments for AMD and RP focus on repairing or replacing these damaged photoreceptor cells.
Methods of Treatment
Several experimental therapeutic treatments have been developed in attempts to counter the effects of these diseases. These therapies include intravitreal (inner eye) injections of growth factors drugs and gene therapy approaches. These avenues, however, only help to inhibit RP and AMD [5]. Thus, they are of little value for patients whose photoreceptor cells have already deteriorated [5]. To address this issue, pPhysicians have also investigated the transplantation of retinal tissue, but have seen little success because of foreign tissue rejection and the difficulty of the surgical procedure [6]. The most recent approach involves reactivating the visual neural system by replacing damaged photoreceptor cells with prosthetic devices. The most recent approach involves the reactivation of the visual neural system through the use of prosthetics. The theory behind these prosthetic models is discussed in the following section.
Theory Behind the Retinal Prosthesis
The Visual Neural Pathway
In order to understand how the prosthesis was conceived, it is necessary to understand how light waves absorbed by the eye are interpreted as sight. This process is called the visual neural pathway. As discussed earlier, light first enters the eye through the cornea and lens and is focused upon the retina. The rods and cones (photoreceptor cells) within the retina then convert light energy into electrochemical signals. These signals propagate through two more layers of the retina that contain bipolar, horizontal, and ganglion neural cells. Finally, the signal is transmitted to the brain via the optic nerve. The key to reversing the effects of AMD and RP lies in reconstructing the damaged visual neural pathway.
Rebuilding the Visual Neural Pathway
It has been well established that retinal neurons may be electrically stimulated to propagate visual signals to the brain [7]. So it follows that the design of a retinal prosthetic device should stimulate retinal neuron, and thereby replace damaged photoreceptor cells.by initiating the transmission of visual signals to the retinal neurons by calculated electrical impulses. Two devices—the first of their kind—are currently FDA approved for clinical feasibility trials: the Artificial Silicon Retina (ASR) developed by Dr. Alan Chow of the Louisiana State University Eye Center and the Multiple-Unit Artificial Retina Chipset (MARC) conceived by Dr. Mark Humayun of the Doheny Retina Institute at the University of Southern California. Both designs are intended to replace damaged photoreceptor cells and re-establish the retinal neural pathway.
The assessments made over the next few years will determine their efficacy and biocompatibility [8].
The ASR and MARC visual prostheses are based on the discovery that the remaining inner retinal neurons in patients with AMD and RD can be electrically stimulated to send visual signals to the brain. These prosthetic devices capitalize on the advances in micro-technology, using high-density integrated circuitry that may be implanted into the epi-retinal and sub-retinal tissue spaces of the eye [9]. Once incorporated into the retinal tissue, both devices are able to induce biological visual signals by sending artificial photoelectric signals to electrically stimulate electrical pulses to specific areas of the retina in response to variations in light and changing images. The ASR and MARC devices differ in the retinal cells they stimulate. ASR is implanted below the retinal layers and excites the bipolar and horizontal cells just above the photoreceptor cells. In contrast, MARC is placed on the surface of the retina and directly stimulates the ganglion cells. Although they are implanted in different locations within the retina, both ASR and MARC produce the same effect by initiating signals in the visual neural pathway.
By replacing the normal electrochemical signals of photoreceptor cells with artificial electrical signals, both ASR and MARC may be able to rebuild the neural pathway that has been damaged by AMD and RP. This new concept in biotechnology has great potential to reverse the effects of AMD and RP and restore vision in those patients who have received unsuccessful treatments in the past.
Structural Design
Because the design of the ASR and MARC prosthetic devices are similar, the structural components of ASR will be focused upon in this section. The ASR microchip is a circular disk, 2 mm in diameter, and 25 mm thick—less than the thickness of a single human hair. The ASR and MARC prosthetic devices are similar in design, differing in the manner by which light is converted into electrical signals. ASR uses microphotodiodes, which are essentially solar cells that convert light energy into electrical current. MARC uses a more complicated system that processes images taken by an external camera and produces electrical current on certain areas of the microchip.
Both microchips are about as thin as a sheet of paper and only several square millimeters in size. Their foundations are composed primarily of a silicon substrate, chosen because of its extremely low risk of bodily rejection [10]. The foundation of the microchip is composed primarily of silicon substrate [5]. Silicon was chosen because of its rare instances of bodily rejection, meaning there are very few biocompatibility issues [10]. Affixed to the silicon base areis approximately 5,000 microscopic solar cells, called microphotodiodes, which are separately connected to their own stimulatingan array of stimulating electrodes. The array of electrodes is comprised of several different materials including silicon nitrate, titanium, and platinum. Platinum is commonly used in all neural stimulation applications because of its compatibility with human tissue and high electrical conductivity [11]. The use of biocompatible materials technology within these two implantable devices has propelled the success of ASR and MARC, as has the size. Small form factor contributes to lower power requirements and more precision in the placement of the device.
Surgical Procedure
The microsurgical procedure involves a standard ophthalmic operation called a vitrectomy and retinotomy with an additional step for the sub-retinal implantation of the ASR chip [12].
- Vitrectomy—Three tiny incisions are made in the white part of the patient’s eye. The operating instruments will be inserted through these openings.
- Retinotomy—The vitreous gel that fills the eyeball is replaced by a saline solution.
- Implantation—A small incision is made in the retina about 20Å above and to the side of the macula region. Saline is injected into the retina in order to create a small pocket in the sub-retinal space where the ASR microchip will be implanted. After the chip is placed inside the pocket and positioned correctly, air is introduced into the eye to gently push the retina back down. (Over the period of two weeks, the air bubble and saline solution are absorbed and replaced by vitreous gel.)
Clinical Trials
Since their January 2000 FDA approval to conduct a safety and feasibility trials, the ASR and MARC microchips have been implanted in ten 20 patients who were rendered legally blind by RP and AMD. So far, both Dr. Chow and Dr. Humayun report that their devices is producing lasting visual improvements among the patients. Participants report improved visual acuity, contrast perception, and increases in visual-field size. Some patients are even capable of seeing letters on standard optometric ETDRS charts [13]. In addition, none of the patients has shown signs of implant rejection, infection, or any other biocompatibility issues. Though only partial vision has been restored in the patients, these experiments mark the first time where progress has been made in reversing the effects of AMD and RP. The success of these trials has given hope to those who suffer from these debilitating diseases. Dr. Chow and Dr. Humayun are confident that their retinal prostheses will prove their long-term safety, efficacy, and suitability [13]. They are both very optimistic, saying that, in the future, these devices will be able to completely restore vision in AMD and RP patients. However, Dr. Chow made it clear that the long-term safety, efficacy, and suitability of the ASR implant have yet to be determined [13].
Future Impact and Conclusion
Because of the advancements made in biomedical engineering, individuals suffering from the blinding effects of Age-Related Macular Degeneration and Retinitis Pigmentosa now have hope that one day they may be able to see again. The innovations in silicon chips and microcircuitry as well as the discovery that electrical stimulation can excite retinal neurons have given ophthalmologists the means to create prosthetic devices to replace damaged retinal cells. The two newest retinal prostheses, ASR and MARC, re-establish the visual neural pathway by replacing the neural cells damaged by AMD and RP. These devices have shown great promise in restoring vision to patients, and as research continues, physicians and scientists are confident that the retinal prosthetics will one day Although the devices currently restore only partial vision, it is expected that continued success with clinical trials and research will aid physicians in completely reverseing the affects of retinal disease. Clearly, the advent of these retinal prosthetics has made the impact of biomedical engineering more deeply appreciated.
References
[1] N. Campbell, J. Reece, and L. Mitchelle. Biology. Menlo Park: Benjamin/Cummings, 1999, pp. 1998.
[2] “Optobionics.” Internet: www.optobionics.com, [Oct. 20, 2003].
[3] S. Parmet. “Age-Related Macular Degeneration.” Journals of the American Medical Association. vol. 288, pp. 2358-9, 2002.
[4] “Retina Source.” Internet: www.theretinasource.com, [Oct. 19, 2003].
[5] N. Peachey, A. Chow. “Subretinal Implantation of Semiconductor-Based Photodiodes: Progress and Challenges.” Journal of Rehabilitation Research and Development, vol. 36, iss. 4, pp. 381-91, 1999.
[6] R. Aramant, M.J. Seiler, and S.L. Ball. “Successful Cotransplantation of Intact Sheets of Fetal Retina with Retinal Pigments Epithelium.” Invest Ophthalmol Vis Sci., vol. 40, pp. 1557-64, 1999.
[7] M. Humayun. “Intraocular Retinal Prosthesis.” Trans Am Ophthalmol Soc., vol. 99, pp. 271-300, 2001.
[8] “Microelectronic Retinal Implants.” American Academy of Ophthalmology, [On-line]. Available: http://www.aao.org/education/library/rapid/retinal.cfm, 2000. [Oct. 4, 2003].
[9] R. Lakhanpal, et al. “Advances in the Development of Visual Prostheses.” []Current Opinion in Ophthalmology[/i], vol. 14, no. 4, pp. 122-7, 2003.
[10] S. Shaikh, et al. “The Effect of Silicone Ocular Surgical Devices on Serum IgG Binding to Silicones.” Am J Ophthalmol., vol. 126, pp. 198-804, 1998.
[11] J.D. Weiland, D.J. Anderson, and M.S. Humayun. “In Vitro electrical Properties for Iridium Oxide Versus Titanium Nitride Stimulating Electrodes.” IEEE Trans Biomed Eng. , vol. 49, pp. 1574-9, 2002.
[12] L. Charters. (2003) “Microelectronic Device Continues to Show Promise.” Ophthalmology Times. [On-line]. Available: http://www.ophthalmologytimes.com/ophthalmologytimes/article/articleDetail.jsp?id=24606 [Oct. 22, 2003].
[13] N. Groves. (2003). “Subretinal Device Improves Visual Function in RP Patients.” Ophthalmology Times. [On-line]. Available: http://www.ophthalmologytimes.com/ophthalmologytimes/article/articleDetail.jsp?id=65335 [Oct. 22, 2003].