Abstract:
We often think of art and engineering as two distinct concepts – maybe even opposites. But the reality is that they are not mutually exclusive. In fact, in the world of fresco painting, chemistry and art intertwine to produce masterpieces that withstand the test of time and contribute to a rich global culture. The scientific secret of the fresco lies in the plaster used to prime the surface on which the artist paints. The proper making and usage of the special plaster employs concepts such as chemical bonding, porosity, diffusion, and solubility in order to ensure a vibrant and durable painting for years to come. Differences in the technique, such as the ‘buon fresco’ and a ‘fresco secco’ produce different results, but the desirability of each method depends on the goals of the piece, just as an engineer optimizes an industrial process based on the resources available and the performance goals.
The Art of Science: An Introduction
You’ve probably heard of the grand masters of Renaissance art like Michaleangelo, da Vinci, and Raphael – if not by name, perhaps by their great masterpieces such as da Vinci’s The Last Supper which depicts Jesus eating wine and bread with the twelve apostles in dull hues, or Raphael’s The School of Athens, a bright and colorful depiction of a gathering of great minds in a grand hall. If these don’t ring a bell, perhaps you’ve seen the popular image of Adam’s limp hand reaching out to touch God’s in The Creation of Adam by Michaelangelo. Many are familiar with the masterful paintings created during the Renaissance Period in one way or another. However, what many people don’t know is the amount of labor, planning, and engineering that went into the creation of a single masterpiece from this era. Yes, you read that right – engineering.
I was first introduced to the science of art when I visited The Last Supper in Milan, and my tour guide explained that the painting had withstood the test of time because of the chemical bonds that are created when painting with the ‘fresco’ technique. At the time, I was a high school chemistry nerd and an artist, so my interest peaked. In my subsequent travels to artistic landmarks around Europe, I must have viewed hundreds of fresco paintings. What surprised me each time was the age of these works, and how intense the colors and details still looked after all these years. In compositions of people, even the folds of their clothing and facial intricacies remain intact. What surprises me even more today as an engineer is the sophisticatedly designed and laborious processes for prepping, planning, and execution of the works.
The Terminology: Buon Fresco and Fresco Secco
So, what was so special about the chemistry of the fresco technique that allowed it to become the technique of choice for the grand masters of the Renaissance? Before going into chemical territory, it’s important to understand that there are two main fresco techniques, and the one utilized determines the quality and durability of the painting, as well as how the chemistry plays out. The first type is the ‘buon fresco’, which means ‘true fresh’ in Italian. This is the preferred technique as the end result is highly durable and vibrant.
The ‘fresco secco’, or ‘dry fresh’ in Italian, is also good but does not yield the same levels of durability and vibrancy as the buon fresco [1, 2, 3]. This is why several of the most famous frescos, like the entire interior of the Sistine Chapel, were done using the buon fresco technique.

Fig.1: Buon Fresco: Photograph of The Ceiling of The Sistine Chapel painted by Michelangelo [1]

Fig. 2: Fresco Secco: Photograph of The Last Supper painted by Leonardo da Vinci [4]
Notice the superiority in the vibrancy and texture of Fig.1, a buon fresco, compared to that of Fig.2 a fresco secco. After having viewed both of these paintings in person, I can confirm that the contrast in quality shown in the images above is true to reality. The Sistine Chapel walls are level and smooth. They glow with bright yellows, blues, reds, and greens, and even though the paintings are several meters above your head, the creases of clothes, expressions, and shadows of muscle are clearly discernible. The Last Supper is a masterpiece as well, but even after restorations, the surface of the work remains rough, and the colors dull. Even the reds and blues, two of the brighter colors used in the piece, are matte and nonuniform, leaving what the painting must have looked like before its deterioration up to our imagination.
Plaster Preparation: The Lime Cycle
Like any proper work of engineering, the most important part is a solid foundation. A buon fresco is no exception. The durability of the painting is achieved through three layers of plaster that prime the working surface. Priming is the act of preparing the surface so that when the paint is applied, some kind of attachment is formed that allows the paint to ‘stick’, or in this case, chemically bond, to the surface.
Reaction I
The plaster is created using a material called quicklime. Quicklime is made by taking limestone, a sedimentary rock found in the earth around warm coastal areas, and calcifying it. The calcination of limestone happens when limestone is heated up to temperatures around nine hundred degrees celsius, resulting in the reaction that turns calcium carbonate (limestone) into calcium oxide (quicklime) and generates carbon dioxide [5]:
CaCO3 (s) + heat CaO (s) + CO2 (g)
Rewriting the chemical equation above in common terms:
limestone solid + heat from energy source ->quicklime solid + carbon dioxide gas
Reaction II
When the calcium oxide is dissolved in water, the solvent used for the plaster creates a highly exothermic reaction. Heat is released due to the formation of new bonds between the quicklime and the water molecules. From the reaction below, calcium hydroxide, or slaked lime, is produced in a solution called limewater:
CaO (s) + H2O (l) Ca(OH)2(aq) + heat
Rewriting the chemical equation above in common terms:
quicklime solid + liquid water->slaked lime dissolved in water + generated heat
Reaction III
The next step is adding an aggregate. An aggregate is a substance in a solution that does not fully dissolve but instead forms suspensions of molecules [6]. Basically, it’s like adding bigger particles into smaller particles to form a mixture. In the case of fresco plaster, the bigger particles are typically sand. Although, during Michaelangelo’s painting of the Sistine Chapel, he had the idea to use volcanic ash as an aggregate instead due to a quicker drying time that would prevent mold formation, and because it allowed for a smoother spread of the plaster for painting on [7, 8].
The significance of adding an aggregate to the limewater is that it allows for the creation of gaps within the plaster mixture. This means that the plaster layers will be porous, allowing for carbon dioxide from the air to diffuse into these gaps and react with the dissolved slaked lime to form a durable calcium carbonate solid [7]. However, this reaction is endothermic, requiring energy to break the bonds in the slaked lime. That required energy actually comes from the initial reaction of quicklime and water, as it will still be producing heat that can push this final reaction forward until the plaster dries out:
Ca(OH)2(aq) + CO2 (g) + heat CaCO3 (s)+ H2O (l)
Rewriting the chemical equation above in common terms:
slaked lime dissolved in water + carbon dioxide gas ->limestone solid + liquid water
Notice that this reaction returns the plaster to its original component, limestone. This series of reactions, as depicted in Fig. 3, is called the limestone cycle [9]. The last reaction is the most important, as the painting must take place on a fresh, wet layer of plaster so that when the layer dries, and the limestone is created, the pigments become trapped within the stone itself. This is the step responsible for the persistence of buon frescoes over time, as limestone is a very chemically stable compound.
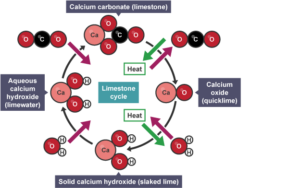
Fig.3: Diagram of the Limestone Cycle [9]
Laying The Foundation: Scratch, Arricio, and Intonaco
So, once the plaster is made, how do the layers form a foundation for the fresco? The first layer, the scratch layer, is meant to prepare the wall and serve as a binder between the wall and the subsequent layers. It is composed of a higher proportion of aggregate to lime. Once this layer dries, the next layer, called the ‘arriccio’ is applied. The arriccio contains more lime than the aggregate. When the arriccio dries, it is on this layer that the artist will create a sketch called the ‘sinopia’ of the piece.
For example, if an artist desired to paint a horse, the sinopia would be a rough outline of its body drawn onto the arriccio. This serves as a blueprint, eventually covered by the last layer of plaster, the ‘intonaco’. The artist will paint on the intonaco [11, 12].
For large pieces, the artist may work in parts. This means that intonaco is plastered onto the arriccio in small sections, each called a ‘giornata’, or “a day’s work” in Italian. Working in small sections allows the artist to complete the giornata before the intonaco dries and turns into limestone [1]. Quick completion is key, as the potential for the paint to diffuse deeper into the layer is greater when fluid is still present in the intonaco.
Applying this concept to the horse painting example, the artist might plaster on the intonaco for a small section of the horse’s body – let’s say the head. This would be one ‘giornata’. On this giornata, the artist would need to first redraw the sketch of the horse’s head based on the surrounding sinopia of the horse’s body. This is tedious and inefficient, as the artist also needs to be able to finish painting the horse’s head in full color that day. The following day, the artist may move onto the horse’s neck, and so on.
Time Optimization: Diffusion and the Cartoon Method
Diffusion of the pigment into the intonaco layer begins when the paint on the artist’s brush meets the wet plaster. The paint then physically separates from the brush onto the intonaco due to parallel stress, also known as shear, from the brush stroke. Diffusion of the paint into and through the plaster occurs due to a mass transfer concept called capillary action, which is the movement of liquids through narrow spaces due to the effects of adhesion and cohesion. In simple terms, the fluid in the paint is attracted to itself, so it stays together (cohesion), but it’s also attracted to the fluid in the plaster, so it’s pulled deeper into the layer (adhesion) [13]. The capillary movement, in this case, is induced by the perpendicular stress, or force, of the paintbrush pushing the paint into the intonaco, which forms capillary networks within the layer as the intonaco continues to turn into limestone and dry, the pigments from the paint become trapped within the strong crystalline structure of limestone that is formed [14].
The inefficient sinopia method was later replaced during the Renaissance period by the ‘cartoon’ method, which entailed drawing the sketch on a cloth veil instead of the arriccio layer. The veil was then placed over the intonaco for the day, and holes were poked through it, tracing the sketch. This served as a sort of stencil on which colored powder known as ‘spolvero’ was dusted [15]. This imprinted the sketch on the intonaco itself, making the visualization and completion of the giornata easier and more efficient. The quicker the artist could work, the more durable the paintings could be since penetration and diffusion of the paint pigments could be maximized.
Returning to the horse painting example, the cartoon method would help in the sense that there would be no need to ‘redraw’ the sketch of the horse’s head for the giornata. Instead, the veil with the horse sketch would simply be lowered onto the giornata, poked, dusted, and lifted – and voila! The artist can now start the true painting. This reduces the amount of guessing an artist needs to do regarding the accuracy of the image, and thereby reduces time worked on the giornata.
An Alternative: The Fresco Secco
The fresco secco technique is an alternative to the buon fresco. Unlike the buon fresco, fresco secco is done on dry plaster. In preparation of the painting, only the surface of the intonaco is moistened in limewater. This means that the pigments in the paint do not penetrate the intonaco layer, but instead remain in a very thin, superficial crust of limestone formed by the limewater and the air [1, 2]. This makes the fresco secco more prone to flaking and dulling over time.
Additionally, fresco secco requires that the dry plaster be sanded in order to produce a rough texture that makes it easier for the paint to stay on the surface [1]. The paint itself will also require the addition of a binding component. The most common binder for the fresco secco technique is egg yolk [16]. The yolk is favored because it somewhat reduces the adverse effects of aging such as yellowing and wrinkles [17, 18]. This is due to the antioxidant properties in egg proteins that combat paint oxidation.
As previously mentioned, fresco secco has inferior durability to the buon fresco, so why would anyone want to use it? The answer is ease and time. Because the technique is done on dry plaster, there is no rush in the painting process. This gives artists the flexibility to start and stop a piece as they please, whereas with buon fresco, it is critical to complete the giornata while wet. Correcting mistakes is also much easier when using the fresco secco method, as one can simply overpaint. With buon fresco, mistakes must be corrected by scraping off the intonaco layer and starting it from scratch since the paint is encapsulated by the intonaco layer itself. Many buon frescoes, such as Raphael’s The School of Athens, used the fresco secco technique for the finishing details of a piece, as this lowered the risk of making mistakes and was easier to implement [1, 2, 19].
Appreciation for the Engineers of the Fresco
The grand fresco masterpieces that have endured through history represent incredible achievements in both science and engineering. Artists who undertook the challenge of fresco painting may not have initially seen themselves as engineers, but the parallels between their creative processes and engineering principles are undeniable. Like engineers, fresco artists had to carefully choose their methods, establish a strong foundation for their work, and continually refine their processes to achieve optimal results. By understanding this, we can gain a deeper appreciation for the diligent thought and labor that went into their pieces. Had it not been for the unwavering dedication of past fresco artists, we might never have had the privilege of experiencing the wonder of being surrounded entirely by color in the Sistine Chapel or being blown away by the immense detail present in the likenesses of historical figures. Thanks to the engineers of art, we can experience the past vividly.
References
[1] I. Meyer, “Fresco Painting – The Age-Old Art of Applying Paint to Plaster.” artincontext.org. https://artincontext.org/fresco-painting/ (accessed Oct. 9, 2023)
[2] Britannica, “fresco painting.” britannica.com. https://www.britannica.com/art/fresco-painting (accessed Oct. 9, 2023)
[3] F. Crowninshield, “MURAL PAINTING.1–IX: DURABILITY OF FRESCO.–ITS PRESENT POSSIBILITIES.–FRESCO COMPARED WITH WAX–PAINTING.–FRESCO–SECCO. FRESCO–SECCO,” The American Architect and Building News (1876-1908), vol. 19, no. 537, p. 171–, 1886.
[4] A. Zelazko, “Last Supper painting by Leonardo da Vinci.” britannica.com. https://www.britannica.com/topic/Last-Supper-fresco-by-Leonardo-da-Vinci (accessed Oct. 16, 2023)
[5] A. Moropoulou, A. Bakolas, and E. Aggelakopoulou, “The effects of limestone characteristics and calcination temperature to the reactivity of the quicklime,” Cement and concrete research, vol. 31, no. 4, pp. 633–639, 2001, doi: 10.1016/S0008-8846(00)00490-7.
[6] LibreTexts Chemistry, “Aggregate Particles in Aqueous Solution.” chem.libretexts.org. https://chem.libretexts.org/Bookshelves/General_Chemistry/Book%3A_General_Chemistry%3A_Principles_Patterns_and_Applications_(Averill)/13%3A_Solutions/13.07%3A_Aggregate_Particles_in_Aqueous_Solution (accessed Oct. 9, 2023)
[7] Artrageous with Nate. Michelangelo & The Science of Fresco Painting | Chemistry Meets Art. (May 27, 2016). Accessed: Oct. 9, 2023. [Online Video]. Available: https://www.youtube.com/watch?v=lUddM_Y_snQ
[8] J. Katz, “The Measure of Genius: Michelangelo’s Sistine Chapel at 500.” smithsonianmag.com. https://www.smithsonianmag.com/arts-culture/the-measure-of-genius-michelangelos-sistine-chapel-at-500-123313873/ (accessed Oct. 9, 2023)
[9] Science Learning Hub, “Lime — a time-tested chemical.” sciencelearn.org.nz. https://www.sciencelearn.org.nz/resources/474-lime-a-time-tested-chemical (accessed Oct. 9, 2023)
[10] BBC BITESIZE, “The limestone cycle – Limestone.” bbc.co.uk. https://www.bbc.co.uk/bitesize/guides/z6gqmsg/revision/2 (accessed Oct. 14, 2023)
[11] D. Meyernik, “Buon Fresco: Materials & Techniques.” traditionalbuilding.com. https://www.traditionalbuilding.com/product-report/buon-fresco-painting (accessed Oct. 9, 2023)
[12] The National Gallery, “Fresco.” nationalgallery.org.uk. https://www.nationalgallery.org.uk/paintings/glossary/fresco (accessed Oct. 9, 2023)
[13] LibreTexts Chemistry, “Capillary Action.” chem.libretexts.org. https://chem.libretexts.org/Bookshelves/Physical_and_Theoretical_Chemistry_Textbook_Maps/Supplemental_Modules_(Physical_and_Theoretical_Chemistry)/Physical_Properties_of_Matter/States_of_Matter/Properties_of_Liquids/Capillary_Action (accessed Oct. 9, 2023)
[14] M. Horgnies, M. Bayle, E. Gueit, E. Darque-Ceretti, and M. Aucouturier, “Microstructure and Surface Properties of Frescoes Based on Lime and Cement: The Influence of the Artist’s Technique: Microstructure and surface properties of frescoes,” Archaeometry, vol. 57, no. 2, pp. 344–361, 2015, doi: 10.1111/arcm.12093.
[15] C. Green, “How Buon Fresco Brought Perspective to Drawing.” daily.jstor.org. https://daily.jstor.org/buon-fresco-brought-perspective-drawing/ (accessed Oct. 9, 2023)
[16] LibreTexts Humanitites, “Painting.” human.libretexts.org. https://human.libretexts.org/Courses/Prince_George%27s_Community_College/Introduction_to_Art_and_Art_History_Part_I/04%3A_Media_and_Processes/4.02%3A_Painting (accessed Oct. 9, 2023)
[17] O. Ranquet, C. Duce, E. Bramanti, P. Dietemann, I. Bonaduce, and N. Willenbacher, “A holistic view on the role of egg yolk in Old Masters’ oil paints,” Nature communications, vol. 14, no. 1, pp. 1534–1534, 2023, doi: 10.1038/s41467-023-36859-5.
[18] J. Prisco, “Scientists identify secret ingredient in Leonardo da Vinci paintings.” cnn.com. https://www.cnn.com/style/article/old-masters-da-vinci-egg-yolk-painting-scn/index.html (accessed Oct. 9, 2023)
[19] “The School of Athens.” projects.mcah.columbia.edu. https://projects.mcah.columbia.edu/raphael/htm/raphael_athens_tech.htm (accessed Nov. 28, 2023)


