The enriched lifestyle that many Americans enjoy is largely dependent on petroleum. Oil and natural gas provide 65% of our energy needs and 97% of our transportation fuels. Everyday items such as ink, heart valves, telephones, rubbing alcohol, sports car bodies, shampoo, and cosmetics are all products of petroleum. These products are produced through a method of petroleum refining in which the petroleum is separated into its different components based on their boiling points. The connection between crude oil and these daily-used products becomes apparent once one understands how petroleum is generated and refined.
Petroleum Is Everywhere
Consider the typical morning routine of an average college student. The annoying buzz of an alarm clock blares at nine and if, after much debate, the student actually decides to get up for class, she crawls out of bed, flips on the light, takes a shower, puts in her contact lenses, puts on her new outfit (made primarily of polyester), dries and curls her hair, puts on her make-up and is ready to leave. By the time she is done getting ready, it is about 9:59 a.m. (thanks in part to the fact that Sally and Maury are on the television at this time) and she must speed to campus in her red convertible sports-car. Just as she arrives at the campus and is about to park, a caller to the talk-show on the radio station is complaining about an oil company seeking to drill for oil and gas in his city. This is all the student hears before rushing away to class. She agrees with the man because she believes oil companies destroy the environment and pollute the water and land. However, by reaching such a conclusion the student would only be understanding one side of the story.
Thousands of petroleum products are used and taken for granted each day by people all over the world. Consider our student of interest and discover some of the petroleum products used in her hour of preparation. The student is awakened by a petroleum product (her alarm clock), is wearing petroleum (her pajamas), and uses petroleum to turn on the light (the light switch). She has a convenient shower thanks to petroleum (the water pipes and shower curtain), washes her hair with petroleum (shampoo), and then applies petroleum to make her skin soft (lotion). Next, the student inserts soft contact lenses and uses a contact lens case, hair curlers, lipstick, deodorant, perfume, a comb, and toothpaste, all of which are petrochemical products. During the hour, she also watched the television, which is made in part from petroleum. On the way to school, she drove in petroleum (the body of her sports-car) and used petroleum to get there (fuel) (see Fig. 1).
The American economy is strongly tied to the success of the oil industry. In addition to its use in the manufacturing of the products mentioned above, petroleum is collectively the largest source of energy in the United States. Oil and natural gas provide 65% of our energy needs. Natural gas provides 25% of the energy Americans consume and is used for many conveniences, from heating and cooking to making electricity. It is even a source of power for some automobiles. According to the American Petroleum Institute, oil provides 40% of our energy and 97% of our transportation fuels. In December, 1998, American oil consumption was 19.284 million barrels of oil per day, and this number is increasing each year. The United States consumed 18.2 million barrels of oil a day in 1996. Although petroleum has an immense impact on our daily lives, it often goes unnoticed. The average American is not likely to see the connection between crude oil (a smelly, messy, dark-brown, slimy substance) and a red, convertible, sports-car. The connection can more easily be seen if one understands what petroleum is and how it is generated and refined.
Where Does Petroleum Come From?
Petroleum is a limited resource. As the total production rises, the difficulty in obtaining the oil rises as well. This is largely due to the process of petroleum generation. Petroleum is an elaborate mixture of organic compounds, consisting mainly of alkanes and aromatic hydrocarbons.
Hydrocarbons are molecules composed of carbon and hydrogen atoms arranged in a variety of ways. Alkanes are hydrocarbons containing carbon-carbon single bonds, and aromatic hydrocarbons contain a fragrant carbon ring. Hydrocarbons are made from decomposing organic material such as microorganisms. These organisms, such as phytoplankton and zooplankton, are very concentrated in oceans and lakes. Microorganisms die in oceans and lakes and settle to the bottom, where they are rapidly buried with clays, silts, and mudstones. These fine-grained sediments are deposited in layers of shale and mudstone, making up sedimentary basins. This rapid burial prevents the organic material from decomposing, and a limited amount of oxygen prevents the material from being oxidized.
The generation of hydrocarbons is a very slow process which occurs when the temperature ranges from 150 to 300 degrees Fahrenheit. Over a long period of time, the burial depth increases, thereby increasing both temperature and pressure. Every 100 feet of burial depth generally results in a temperature increase of 1 to 5 degrees Fahrenheit. Methane gas is generated when the burial depth is up to 5,000 feet, and between 5,000 and 21,000 feet is called the “oil window.” The oil window is the subsurface region where oil and gas generation occurs. This is also the region of appropriate temperature for hydrocarbon generation (between 150 and 300 degrees Fahrenheit) [1].
Refining Crude Oil
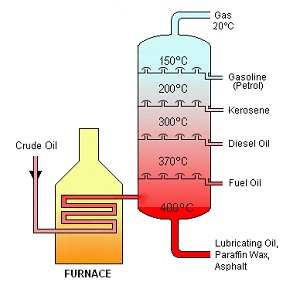
Oil obtained from the ground is referred to as “crude oil.” Crude oil goes through a process of refining in which it is converted to the thousands of products on which people depend daily. The main operation in the refining process is called distillation. Distillation is a separation process in which the various components of the crude oil are separated based on their different boiling points (see Fig. 2). The distillation process begins by pumping crude oil into a furnace in which it is heated to about 400 degrees Celsius (752 degrees Fahrenheit). The first hydrocarbons to escape are those with the least amount of carbon atoms and the lowest boiling-point temperature. This takes place at approximately 40 degrees Celsius (104 degrees Fahrenheit).
At this temperature, the hydrocarbons boil and turn into vapor. The vapor rises and is then collected on a tray where it is turned back into a liquid. This substance is known as liquefied petroleum gas and is used in making items such as propane, motor fuels, and petrochemicals. Petrochemicals are the raw materials used in making daily household items such as plastics, fabrics, medicines, sweeteners, and cosmetics. The next substances to boil are those whose boiling points are above 40 degrees Celsius [1]. They are called “light distillates” and are used in the production of gasoline, paint solvents, kerosene, jet fuel, and home heating fuel. Hydrocarbons that contain sixteen to twenty carbon atoms boil at temperatures exceeding 300 degrees Celsius (572 degrees Fahrenheit). These heavy distillates are used to produce lubricating oil, grease, and wax. Residues at the very bottom which do not boil off are used to form products such as asphalt, petroleum jelly, and coke [2].
Keeping Petroleum Around
Petroleum and its byproducts clearly affect our lives in a wide variety of ways. After reading this paper, the afore-mentioned college student became aware of her personal dependence on petroleum and began to appreciate this limited resource. She started doing her part to ensure the future existence of petroleum by recycling the used oil from her sports-car. More than 40 million gallons of used oil have been collected since 1991 [3]. Reusing oil reduces the need for oil imports and conserves energy. Two gallons of used oil can generate 36 kilowatt hours of electricity. This is equivalent to the amount needed to run the average household for a day, cook 48 meals in a microwave oven, blow dry hair 216 times, vacuum a house for 15 months, or run a television set for 180 hours [4]. Nearly 10,000 drop-off centers nationwide help motorists dispose of used oil. The oil is recycled as industrial fuel and can be re-refined into lubricants or fuels. The U.S. oil and natural gas industry spent nearly ten billion dollars on environmental protection in 1995. This is two billion dollars more than the annual budget of the U.S. Environmental Protection Agency and is almost equal to the amount spent drilling for new oil and natural gas in 1995 [5].
In conclusion, the oil industry is largely responsible for the quality of life currently enjoyed by Americans. The morning “visit” to the college student illustrates just how much petroleum influences our daily lives. One can be assured that observing the rest of her day would not reveal any decline in the amount of petroleum products used. Petroleum is a valuable resource on which we rely daily and cannot afford to lose.