Total hip replacement, or THR, is a very common and successful procedure. THR is often performed when wear of the hip joint — due to osteoarthritis, rheumatoid arthritis, avascular necrosis, or a related condition — results in extreme pain. The worn femoral head and acetabulum (the ball and socket respectively) are removed and replaced with prosthetic devices that eliminate pain. The new femoral stem, or prosthesis, is implanted into the femur. This is secured in place through a process using either a cemented or an uncemented bone growth technique. A new ball is secured to the tip of the stem. The hip socket, or acetabulum, is replaced through the use of a shell and liner system that acts as an acetabular cup. This is held into place in the same method as the femoral stem, using cemented or uncemented techniques. The femoral ball and the new acetabulum can then perform in the same manner as a well-functioning hip joint.
Introduction
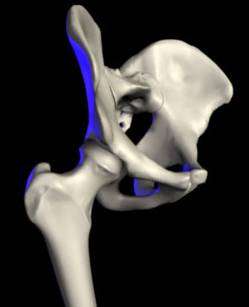
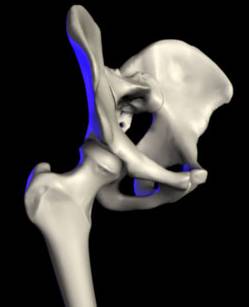
The ability to walk is a treasured aspect of life. Often taken for granted, the hip makes possible many actions, including walking and running. An injured hip can severely restrict a person’s activities and prevent them from leading a full and active life. The hip is a ball and socket joint. The hip is comprised of two main parts: the femur and the pelvis (see Fig. 1). The femur is the thighbone and forms a ball at its tip. The socket, a part of the pelvis, is called the acetabulum. The joint operates with the femoral ball fitting into the acetabulum. A smooth layer of cartilage and a thick lubricant called synovial fluid form a thin layer between the ball and socket, ensuring that the bones are able to move in a nearly frictionless environment. This allows the hip joint to remain stable enough to support the weight of the upper body, unrestricted enough to allow for a full range of leg motion, and virtually friction-free since the well-cushioned ball and socket do not rub together.
If any component of the hip begins to fail, simple acts such as walking become increasingly painful. The most common cause of this joint failure in middle-aged or elderly individuals is osteoarthritis. Osteoarthritis occurs when the cartilage and synovial fluid in the hip joint gradually dissipate due to wear and tear over an extensive period of time. This causes the underlying bone to become exposed. Pain results as the femoral ball and the acetabulum rub directly against each other. Another common cause of hip pain is inflammation of the joints. This can be caused by a variety of conditions, the most common being rheumatoid arthritis, or general inflammation of joints. Inflammation increases pressure within the joint, damaging the cartilage lining of the hip. Rheumatoid arthritis can affect individuals of all ages. Avascular necrosis is another condition that can severely affect proper hip function. This condition is often the result of alcohol abuse. It decreases the blood supply to the hip, which causes the bone around the joint to die from lack of nourishment. Other causes of hip malfunction are trauma to the joint and dislocation of the femoral ball from the acetabulum cup.
The Replacement Procedure
Hip replacement surgery, known commonly as THR (Total Hip Replacement), is now a standard surgical procedure with a high success rate. In the case of an injured hip, the primary damage exists on the upper femoral ball. Surgery begins with the removal of the femoral head. The ball is dislocated from the acetabulum and then removed by cutting through the femoral neck using a power saw. The femoral canal is then prepared so that a prosthetic femoral stem can replace the removed portion of the femur. The core of the mostly hollow femur is dug out in the shape of the metal stem that will serve as the restructured femoral shaft. The new femoral stem is then inserted.
Next, a power drill is used to remove excess cartilage from the acetabulum. The bone of the pelvis is then reshaped in a hemisphere to the exact dimensions of the new, metal acetabular cup. When the size and shape of the drilled out pelvic slot and the new acetabulum component are an exact fit, the prosthetic acetabulum is inserted into place.
The metal ball located at the top of the femoral neck is then inserted into the new acetabulum. While the new hip will not provide as great a range of motion as that of the original hip, it will significantly relieve the constant pain of bone-to-bone contact.
Femoral Implant Details
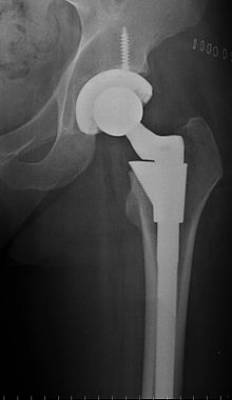
The femoral implant reduces the load on the femur. It commonly comprises two parts, the femoral stem and the femoral ball, though it is available in one piece. The stem portion is composed of a super-alloy metal, usually of a titanium, cobalt chrome, or stainless steel base (see Fig. 2). These metals are extremely hard, allowing them to endure the almost limitless stress that can be exerted upon a hip joint. Their strength and durability make their use common in orthopaedic procedures.
There are two types of fixation techniques: cemented and uncemented. In the cemented implant, bone cement is used to coat the stem as it is placed into the hollow femoral canal. The most commonly used bone cement is an acrylic polymer called polymethylmethacrylate (PMMA). Cemented fixation relies on the formation of a strong mechanical bond between the cement and the bone. Such a bond is generally very durable and reliable, and cemented implantation is widely used.
With the cemented implants, a cobalt chrome or stainless steel stem is used. These stems have a modulus of elasticity, or stiffness, of 200 GPa. This is almost double that of titanium, which has a rating of 100 GPa. The increased stiffness allows more of the load to be placed onto the stem, rather than transferring that load onto the cement and bone. A smooth polished stem is most often used, since the rough or textured surface finishes tend to rub against the cement like sand paper, weakening the PMMA bond. Two to three cm of cement is needed for a proper bond.
Cemented THR, although having a long and distinguished success record, it is not ideal for everyone. This procedure is recommended for less active patients over age 60, younger patients with poor bone quality and density, and individuals with conditions such as rheumatoid arthritis or avascular necrosis. These patients are less likely to put stress on the cement, thereby loosening of the prosthetic stem. Furthermore, these individuals will benefit greatly from the relatively short (approximately one to two weeks) healing time of the cemented option due to their more fragile health.
In some cemented implant cases, fatigue fractures can occur over time that cause the prosthetic stem to loosen and become unstable. The wear debris from cement can generate and cause excess damage to the polyethylene cup, thus generating polyethylene wear. As the body attempts to remove the particles, an inflammatory response can result, possibly causing even further loosening. Cells remove bits of bone around the implant as the inflammation takes place. Known as osteolysis, this condition causes the bone to weaken and instability to occur. The bone loss occurs from the edges of the implant outward, thus resulting in the loosening of the stem.
The alternative to cemented fixation is uncemented fixation. The metal stem is sometimes coated with a porous coating. The non-cemented stems have a coarse and gritty exterior. Research has shown that bone will attach to a metal implant if the surface of the metal has a surface topography that is conducive to attracting new bone growth. The porous coating is generally either pure titanium or a substance known as hyroxyapatite. Hyroxyapatite is derived from the mineral portion of the bone. The coarse and gritty exterior of the stem allows the bone to essentially grow into the surface of the implant. The porous coating, when combined with the surface topography of the stem implant, is conducive to bone growth, allowing it to achieve fixation within the bone.
While equally effective over time, the cementless implants do require a longer healing time than cemented replacements because they depend on new bone growth for stability, rather than just a mechanical bond between the acrylic cement and the bone. In 1870, Wolff’s Law established that bone remodels (i.e. new bone is formed or unneeded bone is destroyed) according to the load it is under. Because of this possible remodeling, the stem must transfer some of the load directly to the bone, in order to ensure bone growth. Titanium alloy stems are used in the uncemented fixation because they reduce this stress shielding, allowing the bone to directly support a portion of the load. Titanium alloy is a better choice than cobalt chrome or stainless steel, with a modulus of elasticity of 100 GPa, which makes it about half as stiff as cobalt chrome and stainless steel. After surgery, up to 12 weeks is needed to satisfy bone growth needs in order to ensure stem stability. This requires the patient to undergo strict weight bearing and movement protection to ensure that a proper bond develops. One possible drawback is that complete pain relief after surgery is not as predictable as with cemented stems. Although most pain dissipates over time, a small but substantial subject patient group still reports pain.
Despite these drawbacks, uncemented replacement remains a viable option, as it can be performed in younger and more active patients, allowing them to eventually return to their active lives. Most surgeries last 10 to 20 years, which means that young patients inevitably have to face revision operations. Non-cemented fixations generally have better longevity and are able to postpone the revisions longer than cemented fixations. Additionally, non-cemented stems help to preserve more bone (since they transfer more load to the bone), which makes revision operations easier. The ball that fits at the top of the implanted femoral head makes up the second section of the completed femoral implant. In early THR procedures, the stem and ball were one solid unit. However, research has shown that there are benefits in making the two pieces modular, or separate, though some stems are still made as one piece. The separation has allowed femoral balls of various sizes and materials to be used. Before the ball is connected to the femoral stem, it is polished smooth to allow easy rotation within the prosthetic socket.
Most femoral balls are now made of hardened metal, either cobalt chrome or stainless steel, or ceramic material. The femoral ball is never made out of titanium, as titanium is too soft, resulting in a bad bearing surface that causes lots of wear. Metal femoral heads are available in diameters of 22, 28, or 32 mm. Larger heads in the 38 to 40 mm range are being studied to decrease dislocations. Recently, ceramic models have been introduced and are made of either aluminum oxide or zirconium oxide. Studies are ongoing in the field of ceramic femoral heads, as the manufacturing of ceramics has vastly improved in the last few years. Recent technology has allowed for a smaller grain size, creating a much stronger material. This type of construction produces low wear, similar to that of their metal counterpart. A difference lies in the thickness of the ceramic models. Ceramic balls are thicker than the metal counterpart as to minimize fracture due to concerns regarding the strength of the material. One drawback of the ceramic models is that they are much more expensive than the metal versions.
Acetabular Implant
The acetabular implant consists of two parts: the shell and the liner. The shell portion is remarkably similar to the construction of the femoral components as previously discussed. In its material construction, there are two options, either a metal alloy base of cobalt chrome or stainless steel. The acetabulum carries with it the same implant techniques, as well as the matching benefits and concerns, of the femoral implant. The primary goal of the shell is to produce a strong bond with the bone. This can be done in the same two ways used to implant the femoral stem. Either a cemented or uncemented implant must take place. The design features aspects such as surface topography. The surgical concerns of surgeon accuracy and bone fixation are identical to that of the previous implantation discussed for the femoral implant.
The acetabular liner is generally made of ultrahigh molecular weight polyethylene. The cup liner is designed to naturally fit snuggly inside the acetabular shell. The inside of this shell fits snugly around the femoral head. It snaps into place in a press-fit model. The ultimate goal of the liner is to create a ball and socket medium that will allow the femoral head a full range of motion while providing a large degree of strength and stability.
Another possible liner is a metal, cobalt chrome or stainless steel, version. Furthermore, there is a “sandwich” version that includes two layers of cobalt chrome or stainless steel, with a layer of polyethylene in the middle. The “sandwich” cup is designed to provide shock absorption in an attempt to reduce wear. A general advantage of the pure polyethylene cups is that they carry with them a lower level of friction than those with metal. The lower friction usually will result in lower levels of wear. However, the polyethylene has also been known to have a greater maximum wear possibility. Although the metal versions have higher friction levels, they carry with them a lower maximum wear level.
Conclusion
Technology has allowed for the constant evolution of new and improved surgical techniques in total hip replacement. Research is continually seeking to perfect implant devices and bonding methods. The advantages of such development are overwhelming. The surgery takes individuals who struggle with the simple action of walking and allows them to once again participate in most ordinary daily activities. It gives these people a second chance to experience life to its fullest.