In recent years, America’s blood shortage has propelled the biotechnology of blood substitutes. Artificial blood does not contain the plasma, red and white cells, or platelets of human blood, but functions to transport and deliver oxygen to the body’s tissues until the recipient’s bone marrow has regenerated the missing red blood cells. Current blood substitutes are either hemoglobin-based oxygen carriers (HBOCs) or perfluorocarbons (PFCs). While HBOCs utilize hemoglobin, an actual component of red blood cells, PFCs rely solely on synthetic chemical processes. Like most technological advances, there are still a number of advantages and disadvantages to consider. In the short term, the prospective benefits of a blood substitute overshadow the shortcomings. In addition to carrying oxygen, such compounds can be sterilized against infectious diseases and used in patients whose religious beliefs prevent them from accepting blood transfusions.
The Crisis Emerges
The word “blood” evokes vivid images of pain and fear. Despite its associations with injury and suffering, blood is inextricably linked with human life. A baby is born with only a cup of blood, yet the average adult body contains ten pints, or twenty cups, of the life-preserving liquid.
Unfortunately, one out of every three individuals will at one point in life not have enough blood to sustain his or her life. For this reason, blood is needed every three seconds, but only five percent of the American population donates blood [1]. These statistics evoke a chilling and frightening reality. What happens when the blood supply, the river of life, runs dry? After the attacks on September 11, 2001, over 500,000 Americans responded to the need for blood donations [1]. Scientists and engineers are replying to the same need, developing a blood substitute to combat the threat of catastrophic blood shortage.
The Search Begins
In response to impending blood shortages, scientists and engineers have begun a quest to discover an ideal blood substitute. Within the human body, blood’s two primary functions are the transportation of oxygen to various tissues and the removal of carbon dioxide from the body [2]. Therefore, a blood substitute need not be a direct, identical replacement for blood. It is instead designed to imitate the oxygen-carrying capacity of the red blood cells. Artificial blood will only serve as a temporary substitute until the recipient’s own body has enough time to reproduce the necessary blood cells to compensate for lost blood [3]. The two principle categories of blood substitutes are known as hemoglobin-based oxygen carriers (HBOCs) and perfluorocarbons (PFCs).
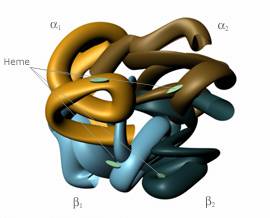
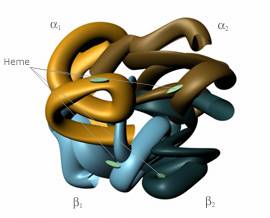
Before one can completely understand the underlying scientific and engineering principles of blood substitutes, he or she must have a basic knowledge of the interaction between oxygen and the blood. In all red blood cells, a molecule known as hemoglobin binds oxygen from the lungs and carries it to the tissues of the body. Structurally, hemoglobin consists of two alpha and two beta chains.
Hemoglobin, shown in Fig. 1, is often referred to as a tetramer, since it is a molecule composed of four units [4]. Each of the alpha and beta chains binds to a heme group, which contains iron. The heme group is responsible for attaching oxygen to the red blood cell [5]. A cofactor called 2,3-diphosphoglycerate (2,3-DPG) is located in all red blood cells, and without it, the hemoglobin could not readily release oxygen to the body’s tissues [3]. However, the outward simplicity of hemoglobin belies its true complexity, for the molecule plays a complicated role in maintaining human life.
Hemoglobin retains its capacity to bind to oxygen even when it is outside of a red blood cell [5]. Therefore, the tetrameric molecule has served as a focal point in the search for a blood substitute. Unfortunately, the answer is neither simple nor apparent, for hemoglobin must be modified before it can be introduced into the body’s circulatory system. This is the consequence of several factors. Hemoglobin is extracted by removing the red blood cell membrane, forming stroma-free hemoglobin [4]. However, the removed hemoglobin lacks 2,3-DPG, which limits the hemoglobin’s ability to deliver oxygen to tissues [5]. In essence, the hemoglobin will bind to oxygen and transport it throughout the body, but without the help of 2,3-DPG, it holds onto the oxygen molecule. An additional problem develops when pure hemoglobin enters the blood stream. The tetramer of four units is rapidly degraded into dimers of two units. The smaller subunits contribute to renal (kidney) toxicity as they are rapidly excreted by the kidneys [3]. While pure hemoglobin intuitively appears to be the ideal blood substitute because of its affinity for oxygen, it must be modified before that concept can become a medical and scientific reality.
From Ingenuity to Reality: the Arrival of a Promising Blood Substitute
Although much of their work remains unnoticed by society, the research of biomedical engineers laid the foundation for blood substitutes. They have designed several promising avenues for blood substitutes, including the use of HBOCs (Hemoglobin-Based Oxygen Carriers). Encapsulated hemoglobin consists of hemoglobin trapped inside artificial red blood cells, where it remains in its tetrameric form. Because the artificial blood cells are synthesized in a laboratory, the surface properties of the cell can be modified to make the blood substitute even more efficient and compatible with the human body (see Fig. 2). In fact, because all blood substitutes lack a red blood cell membrane, they do not contain blood group antigens (which are responsible for determining whether an individual’s blood type is A, B, AB, or O). Thus, cross-matching of blood types before a transfusion is unnecessary. A recent experiment demonstrated that ninety percent of the red blood cells in rats could be replaced with artificial red blood cells [3]. Despite such current discoveries, the process of creating microencapsulated hemoglobin is complicated and requires additional research before it can be introduced into the medical community.
Cross-linked polyhemoglobin is a further example of the advances in the arena of blood substitutes. It entails the use of reagents such as o-raffinose or glutaraldehyde to form cross-links between amino groups (NH2) located on the surface of hemoglobin. Essentially, the process of cross-linking can be defined as the formation of a chemical bond attaching hemoglobin molecules to one another. By the end of the process, four or five hemoglobin molecules are usually joined together [5].
Cross-linking has proven to be highly advantageous in designing artificial blood for the following reasons: the hemoglobin tetramer does not degrade into dimers once it is cross-linked, a 2,3-DPG analog (a molecule that mimics the properties of true 2,3-DPG) can be introduced into the cross-linked molecule, and they are likely to remain in the circulatory system longer than individual molecules of hemoglobin [3]. In essence, the formation of cross-links allows molecules to connect together by holding hands, so the bond is strong enough to prevent the molecules from separating and sturdy enough to allow the addition of 2,3-DPG. The traditional cross-linking reaction paved the way for the development of conjugated hemoglobin. This substance utilizes a cross-linking agent to couple a polymer (a long chain molecule consisting of repeating subunits) to the hemoglobin in order to extend the period of time the blood substitute remains in the body. Through cross-linking, the hemoglobin is able to attach to a polymer instead of another hemoglobin molecule.
Ideas with Flaws: Shortcomings in the Development of HBOCs
In addition to the successful blood substitutes that are becoming viable alternatives to human blood, scientists have pursued flawed methods of creating artificial blood. Their innovative work entails some degree of trial and error, for they cannot determine the success of their work until it has undergone rigorous testing and experimentation. Often, a difference arises between formulations and laboratory results. For example, biomedical engineers attempted to intramolecularly cross-link hemoglobin with chemical reagents. Instead of connecting separate molecules of hemoglobin, the cross-linking occurred between either the two alpha chains or the two beta peptide chains, so the bond was actually formed inside one unit of hemoglobin.
Unfortunately, recent unsuccessful clinical trials (where human patients receive the treatment in order to evaluate its effectiveness, dosage, and side effects) led to the cancellation of several studies of intramolecularly cross-linked hemoglobin [5]. Another method of creating an HBOC introduced the use of recombinant DNA, which arose from the genetic engineering of the bacteria E. coli to produce hemoglobin. As happened with intra-molecularly cross-linked hemoglobin, researchers have abandoned recombinant hemoglobin as a blood substitute. They discovered that it caused severe vasoconstriction, or a narrowing and tightening, of the blood vessel walls [6]. Such failed blood substitutes were viewed as setbacks in the pursuit for the perfect artificial blood, yet engineers continued their research efforts. They realized that, in addition to carrying oxygen, modified hemoglobin could be sterilized against infectious pathogens such as HIV and all forms of hepatitis [4]. This provided them with further motivation to overcome the disappointments and obstacles encountered in research.
The other forms of modified hemoglobin also manifest inherent disadvantages, yet intramolecularly cross-linked hemoglobin and recombinant hemoglobin are the only HBOCs that are no longer topics of current research. For example, even in advanced trials of blood substitutes, all HBOCs exhibit a degree of vasoconstriction like intramolecularly cross-linked hemoglobin. This is caused by hemoglobin’s innate affinity for nitric oxide (NO). In the human body, NO acts as a smooth muscle relaxer and is present in the walls of blood vessels. As the hemoglobin travels through the circulatory system, it picks up NO, thus causing the blood vessels to constrict. This leads to a rapid, dangerous increase in blood pressure [7]. Furthermore, HBOCs are limited to short term usage because they are quickly removed from the circulatory system [5]. Ironically, while HBOCs do not remain in the body for extended periods of time, the storage requirements for artificial blood are much more flexible than the storage requirements of human blood. Human blood must be refrigerated and can be stored for no more than six weeks [3]. Thus, another potential advantage of employing blood substitutes addresses the donor blood shortage crisis.
From an Idea to Production: the Emergence of a Revolutionary Blood Substitute
In today’s society, heart surgery directly or indirectly affects nearly all individuals, yet most are unaware that a blood substitute is often responsible for delivering oxygen to the patient’s body during the procedure. Specifically, a blood substitute known as Fluosol DA has been approved by the Food and Drug Administration for use during coronary angioplasty [5]. It is representative of perfluorocarbons (PFCs), the second class of blood substitutes. Fluorocarbons are straight chain molecules that consist of carbon and fluorine [8]. They are known for their capacity to carry both oxygen and carbon dioxide without actually binding to the gas molecules. Thus, their application extends from solely delivering oxygen to also carrying waste to the lungs for removal from the body [5]. Fluosol DA serves as one of the original PFCs, and it is comprised of 20 percent fluorocarbon [6]. A group of PFCs referred to as the “new perflourochemicals” has recently been developed. They include compounds such as perfluorooctyl bromide (C8F17Br) and perfluorodichorotane (C8F16C12). Such molecular structures of the “new” perfluorocarbons allow the blood substitute to contain a higher concentration of PFCs, which subsequently increases the oxygen carrying capacity of the artificial blood [6]. Through the development and implementation of PFCs, biomedical engineers successfully display their ability to integrate the disciplines of medicine, the sciences, and engineering (see Fig. 3).
Falling Short of Perfection: the Weaknesses of PFCs
Just like HBOCs, however, PFCs do not provide a flawless substitute for blood. For example, in order for recipients to benefit from the oxygen-carrying capacity of the blood substitute, they must be breathing concentrated oxygen. Patients receiving Fluosol DA must be breathing between 70% and 90% oxygen, while individuals given C8F16C12 (a new perfluorochemical) must inhale 100% oxygen. However, normal air only contains 21% oxygen. Additionally, the administration of PFCs can suppress the patient’s immune system. This is attributed to the fact that the reticuloendothelial system (RES) is involved with the removal of PFCs from the circulatory system [6]. By definition, the RES system is composed of cells “responsible for engulfing (phagocytosis) and removing cellular debris, old cells, pathogens, and foreign substances from the bloodstream” [9]. When the cells of the RES are concerned with removing PFCs, they are neither available nor free to attack invading particles such as bacteria and viruses. Therefore, the individual’s body is unable to readily fight off disease.
A Glance into the Future
Although the ideal blood substitute has not yet been developed or marketed, it will serve an integral role in biotechnology’s future. Currently, artificial red blood cells offer an exciting glimpse into forthcoming developments, yet cross-linked hemoglobin will most likely be the first blood substitute with large-scale medical applications [3]. The arena of artificial blood offers a powerful forum where the fields of science and engineering combine to illuminate the ingenuity of mankind. In the quest to find the perfect blood substitute, human innovation and collaboration will address the impending blood shortage.