Alexis Seegan was a junior undergraduate majoring in biomedical engineering and minoring in mathematics in the fall of 2004. She hopes to pursue a graduate career in biomedical engineering and neuroscience, with the long-term goal of becoming an overambitious, overworked, and overpaid college professor.
Engineers at Numotech Inc. and Sandia National Laboratories have designed a device that can reduce the healing period for many different types of wounds including plastic surgery incisions, burns, and necrotizing fasciitis. The most striking feature of the product is its simplicity, which hides the enormous amount of engineering that went into creating it. The Numobag, essentially a plastic bag in appearance, is placed around the wound and then filled with oxygen from an oxygen canister. Using the principles behind hyperbaric oxygen therapy, the Numobag infuses high concentration, high-pressure oxygen into bloodless tissue; this supply of oxygen allows the cells to repair the wound and protect against invading microorganisms. The Numobag allows wounds to receive the healing and antimicrobial benefits of hyperbaric oxygen therapy without an expensive and cumbersome traditional hyperbaric oxygen chamber. Clinical trial data for the Numobag show dramatic decreases in the healing time as well as cost to the patient, and suggest numerous uses for the Numobag outside of wound repair. As the engineers of the Numobag have illustrated, in a society of immense complexity sometimes the simplest solution is the best solution.
Introduction
To most people, a plastic bag, a hair barrette, tongue depressors, and hot glue sound like materials for an arts and crafts project–certainly not for a biomedical device; luckily, the engineers at Sandia National Labs in Albuquerque, N.M., and at Numotech Inc., in Northridge, CA, do not think like most people. Using these household items, a team of engineers from Sandia and Numotech were able to create a working prototype of a novel hyperbaric oxygen treatment system. This prototype, called the “Numobag,” is the culmination of these engineers’ MacGyver-like efforts to develop a new type of treatment for wounds.
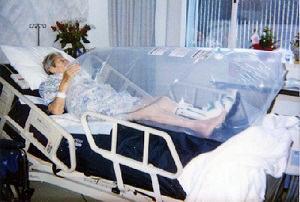
The Numobag consists of a polyethylene bag that slips around the wounded area and applies concentrated oxygen athyperbaric pressures directly to a wound (see Fig. 1). The Numobag requires a canister of oxygen to supply the high oxygen concentration, while a non-electronic sensor attached to the top of the bag describes the pressure inside the bag as “good,” “high,” or “low.” The hyperbaric pressure infuses oxygen into the wound, promotes more rapid healing, and leaves less scarring than standard wound treatment.
Therapy with the Numobag benefits any type of ischemic wound, such as a burn, diabetic ulcer, pressure sore, puncture wound, or even a case of necrotizing fasciitis (flesh-eating bacteria) [1]. However, it is not the use of oxygen therapy for wound treatment that distinguishes the engineers’ design. Rather, the innovation comes from their desire to create a device that makes treatment affordable, less painful, less time-consuming, and more portable for patients suffering everything from third degree burns to diabetic ulcers. In an age where every aspect of society, especially medicine, is becoming increasingly complex, these engineers have created a technology that is overwhelmingly simple, yet incredibly effective.
Oxygen’s Role in Keeping Tissues Healthy
In order to comprehend the value of a portable and disposable hyperbaric oxygen treatment system, it is imperative to understand oxygen’s role in wound repair. Oxygen is a necessary component of cellular metabolism, the process that creates the energy that cells use to build and repair damaged tissue [2]. Any wound that disrupts the normal blood flow decreases the amount of oxygen available to tissues at the site of the wound. The first result of a decrease in oxygen levels (also referred to as hypoxia, a lower than normal pressure of oxygen in the tissues) is compromised cellular function. The decreased amount of energy available to the cells means that they cannot keep up with the tissues’ demands for energy to repair the wound. Oxygen is also required for the production of collagen, a material necessary to heal wounds [3].
Cells near the skin must perform more functions than tissue upkeep and repair, however. The skin is the body’s first defense against invading agents, also called antigens. Once the skin barrier is breached, these cells have to launch an attack on any antigens that make their way into the tissues. Cells called macrophages engulf and eradicate any bacteria or other microorganisms they find in the open tissue. The macrophage destroys the antigens by exposing them to toxic forms of oxygen such as the superoxide anion (O2]) or nitric oxide (NO) [2]. A low level of oxygen therefore also compromises the ability of the tissues to protect against infection.
How Oxygen Helps to Heal Wounds
An ischemic injury decreases the amount of oxygen in the tissues. Hyperbaric oxygen conditions increase the amount of oxygen in the wounded and/or infected tissue, providing the oxygen the cells need to protect and repair themselves [4]. Hyperbaric oxygen therapy exposes the tissue to pure oxygen at a pressure of more than 1 atmosphere, the atmospheric pressure at sea-level [5]. The high pressure forces oxygen to dissolve into the bloodstream; from the blood, the oxygen diffuses into oxygen-starved areas surrounding an injury [6]. A sufficient level of oxygen available enables cellular metabolism to meet the demands of both healing and defending the damaged tissues. The mere presence of an oxygen-rich environment is toxic to some anaerobic forms of bacteria, and hyperbaric conditions can enhance the performance of some antibiotics [3]. In addition to the eradication of infectious agents, hyperoxia stimulates the growth of new blood vessels and an increase in size of existing blood vessels [7]. Moreover, a high oxygen concentration produces more energy for protein production and increases the amount of collagen available; collagen, along with other proteins, can assemble into new capillaries within the wounded area, restoring the blood supply [8].
Clinical trials performed so far with the Numobag have been encouraging. A wide range of patients, from wheelchair-bound individuals with severe pressure ulcers to motorcycle crash victims, has experienced rapid healing with significantly less scar formation than is observed in similar wounds healed without the device [1].
Limitations of Traditional Hyperbaric Therapy
If hyperbaric oxygen therapy is clinically shown to heal wounds effectively, why is it not used more often? One reason is that conventional methods of hyperbaric oxygen therapy are expensive; another is that they are potentially dangerous. A typical treatment involves placing the entire body into a large isolation chamber for 30 to 90 minutes per session, with complete treatment consisting of multiple sessions [5]. This type of treatment cost around $405 per session in 2000, totaling an average treatment cost of $12,000 per patient [5]. Many of the patients suffering from pressure ulcers have conditions that may cause them to recur, such as diabetes or wheelchair confinement, and cannot afford long-term oxygen therapy [1]. Even more problematic than the price of the therapy is the risk patients run of oxygen toxicity to the central nervous system and the pulmonary system while undergoing hyperbaric oxygen therapy. These two factors have largely prevented the conventional treatment from seeing widespread use.
Dr. Madalene Heng, chief of dermatology at the Veterans Administration Center in Sepulveda, CA, wanted to give her patients the healing benefits of oxygen therapy while sacrificing neither their freedom of movement nor their bank accounts [9]. She began to place plastic bags around patients’ injuries and fill them with oxygen, replacing the bulky isolation chamber of traditional hyperbaric therapy. She observed that the risk of oxygen toxicity disappeared as patients no longer needed to have their entire body undergo the hyperbaric treatment [9]. The only problem with Dr. Heng’s device was that she set the internal oxygen pressure based on a subjective measurement of how tight the bag felt, making the treatment difficult to reproduce exactly in different patients. Dr. Robert Felton, a colleague of Dr. Heng’s, felt that his biomedical device company Numotech Inc. could improve the bag by addressing this problem [1].
The Numobag is Born
Dr. Felton faced a number of challenging constraints when designing the new device. Most importantly, all of the parts had to be non-electric because they would be in presence of pure oxygen, which is highly flammable: one spark and the entire device could ignite. Dr. Feltons’s idea was to engineer an external sensor of some sort that would allow nurses or even patients themselves to tell when the bag was at an acceptable pressure. In the past, Dr. Felton worked on projects dealing with pressure wounds that arise as a result of sitting down for long periods of time. He recognized the dire need for a widely available treatment for these wounds, which can often become so severe that they result in amputation. He wanted the product to be inexpensive, disposable, portable, and simple to allow for the patient to use it in their home without the aid of a nurse[1]. Dr. Felton felt these high expectations upon this device were realistic and he set out to achieve them.
Dr. Felton employed Sandia National Labs in Albuquerque, N.M., to help him solve the problem of the pressure sensor. Sandia researchers went against the entropic tide in engineering and looked for a simple solution [1]. The sensor needed to detect the pressure in the bag without electricity, without puncturing the bag itself, and it had to be disposable in order to align with Dr. Felton’s ideals, Dr. Heng’s method of sensing the internal pressure based upon touch worked because the inside pressure is proportional to the tension across the bag’s surface . The phenomenon is akin to blowing up a balloon; the more air put into the balloon, the higher the pressure and the tighter the balloon feels on the outside.
The sensor is based on this same premise that the tension of the polyethylene bag also conveys the internal oxygen pressure [10]. As the bag becomes filled with oxygen, the changing tension of the bag’s surface stretches a spring. This spring, in turn, moves the indicator to reflect the corresponding pressure condition. The marketed Numobag consists of the polyethylene bag with the completely disposable sensor held on with double-sided tape [1]. The researchers developed the prototype of the sensor in Miller’s garage in an attempt to make the sensor as simplistic as possible [9]. A description of this sensor also seems to summarize the project as a whole: “Simple but not easy” (see Fig. 2) [10].
Dr. Felton placed the Numobag in numerous clinical trials before making it commercially available (see Fig. 3). His device allows patients to receive the healing properties of hyperbaric oxygen therapy while minimizing the therapy’s risk, burden, and cost [10]. The Numobag only needs to be applied to the wounded area, lowering the patient’s risk of toxicity and allowing the patient greater mobility [1].
Conclusion
The simplicity of the Numobag’s technology allows it to be affordable; the investment firm backing the Numobag estimates the cost per treatment at $185 [10]. In October 2002, the U.S. government licensed the Numobag, making the device available to the military [10]. The Numobag is currently being used on wounded soldiers in Iraq. Furthermore, the bag provides a much-needed method to heal astronauts injured while in space, as astronauts are prohibited from taking antibiotics while on a mission [1]. The government has developed many uses for the Numobag besides wound treatment, such as a personal isolation chamber in case of a biological attack [10]. Working together, researchers combined their skills in medicine, clinical trials, biomedical engineering, and mechanical engineering to create a deceptively simple, yet remarkable device. Sure MacGyver can escape certain doom with only a pencil and paper clip, but he has nothing on the engineers behind the Numobag.
References
[1] R. Felton. Personal interview. Jun. 20, 2004.
[2] N. A. Campbell and J. B. Reece. Biology. San Francisco: Benjamin Cummings, 6th ed., 2002, pp. 155-902.
[3] Youn, Brian A. “Oxygen and Its Role in Wound Healing.” Environmental Tectonics Corporation. Internet: http://www.etcbiomedical.com/hbo_two.htm, Nov. 3, 1999 [Jul. 13, 2004].
[4] Aydin, Ataken, et al. “Effect of hyperbaric oxygen therapy on nerve regeneration in early diabetes.” Microsurgery, vol. 24, pp. 255-61.
[5] McDonagh, Marian, et al. “Hyperbaric oxygen therapy for traumatic brain injury: A systematic review of the evidence.” Archives of Physical Medicine and Rehabilitation, vol. 85, pp. 1198-204.
[6] Lou, Min, et al. “Therapeutic window for use of hyperbaric oxygenation in focal transient ischemia in rats.” Stroke, vol. 35, pp. 578-83.
[7] Gungor, Atila, et al. “The effectiveness of hyperbaric oxygen treatment in tracheal reconstruction with auricular cartilage grafts (experimental study).” American Journal of Otolaryngology, vol. 24, pp. 390-4.
[8] Vucetic M., P.K. Jensen, and E.C. Jansen. “Diameter variations of retinal blood vessels during and after treatment with hyperbaric oxygen.” British Journal of Opthalmology, vol. 88, pp. 771-5.
[9] Ehrenman, Gayle. “Healing Powers.” Mechanical Engineering Design. [On-line]. Available: http://www.memagazine.org/medes03/healingp/healingp.html, 2003 [Jul. 12, 2004].
[10] Singer, Neal, “Sandia-aided method to heal wounded and diseased achieves US government acceptance.” Internet: http://www.sandia.gov/news-center/news-releases/2002/mat-chem/numobag.html, Oct. 21, 2002 [Jul. 10, 2004].
“Sandia research agreement with Numotech to help create home-use oxygen-healing facility.” Internet: http://sandia.gov/LabNews/LN06-19-98/oxygen_story.html, Jun. 19, 1998 [Jul. 10, 2004].
Sugihara, Atsushi, et al. “The effect of hyperbaric oxygen therapy on the bout of treatment for soft tissue infections.” Journal of Infection vol. 48, pp. 330-3, 2004.